Method for extracting valuable metals from high-iron bauxite with step-by-step acid leaching
A high-iron bauxite and valuable metal technology, applied in the field of mineral processing, can solve the problems of large red mud discharge and low comprehensive utilization rate of valuable elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
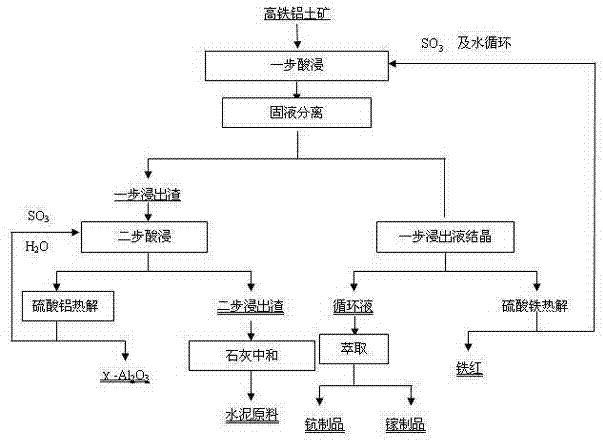
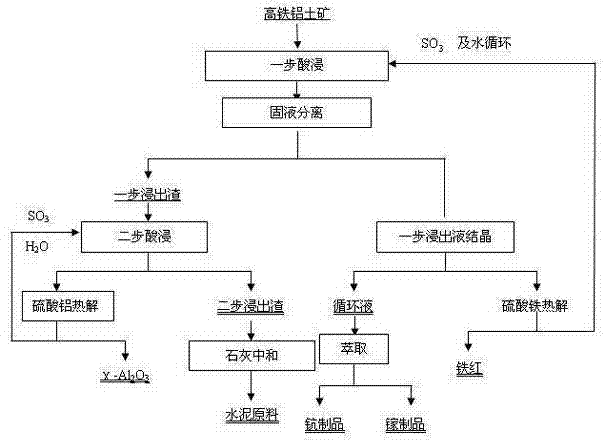
[0036] The high-iron bauxite is crushed and finely ground to a particle size of less than 250 μm, and a sulfuric acid solution with a mass concentration of 50% is used to conduct a one-step leaching reaction under the conditions of a leaching temperature of 120°C, a solid-liquid ratio of 1:3, and a leaching time of 240 minutes. The leaching rate of iron element is 97.05%. One-step leach solution and one-step leach slag are obtained. The one-step leach solution is crystallized to obtain ferric sulfate, and iron red is obtained through pyrolysis;
[0037] After the one-step leaching solution after crystallization is supplemented with acid to a sulfuric acid mass concentration of 30%, the alumina in the one-step leaching residue is leached in a second step under the leaching conditions of leaching temperature 170°C, solid-liquid ratio 1:20, and leaching time 240min. , to obtain the two-step leaching solution and the two-step leaching residue, the leaching rate of alumina in the tw...
Embodiment 2
[0041] The high-iron bauxite is crushed and finely ground to a particle size of less than 74 μm, and a sulfuric acid solution with a mass concentration of 30% is used to conduct a one-step leaching reaction at a leaching temperature of 60°C, a solid-liquid ratio of 1:20, and a leaching time of 60 minutes. The leaching rate of elements is 98.63%, and the one-step leachate and one-step leach residue are obtained, and the one-step leachate is crystallized to obtain ferric sulfate, and iron red is obtained through pyrolysis;
[0042] After adding acid to the sulfuric acid mass concentration of the one-step leaching solution to 60%, the alumina in the one-step leaching slag was subjected to two-step leaching under the leaching conditions of leaching temperature 190°C, solid-liquid ratio 1:10, and leaching time 120 minutes to obtain two One-step leaching liquid and two-step leaching slag, the leaching rate of alumina in the two-step leaching process is 99.45%, the second-step leachin...
Embodiment 3
[0046] The high-iron bauxite is crushed and finely ground to a particle size of less than 144 μm, and a sulfuric acid solution with a mass concentration of 10% is used to conduct a one-step leaching reaction under the conditions of a leaching temperature of 100°C, a solid-liquid ratio of 1:5, and a leaching time of 120 minutes. The leaching rate of elements is 99.16%, and the one-step leachate and one-step leach residue are obtained, and the one-step leachate is crystallized to obtain iron sulfate, and iron red is obtained through pyrolysis;
[0047] After adding acid to the sulfuric acid mass concentration of the one-step leaching solution to 60%, the alumina in the one-step leaching slag was subjected to two-step leaching at a leaching temperature of 200°C, a solid-liquid ratio of 1:5, and a leaching time of 240 minutes to obtain a two-step The leaching solution and the second-step leaching residue, the leaching rate of alumina in the second-step leaching process is 98.17%, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com