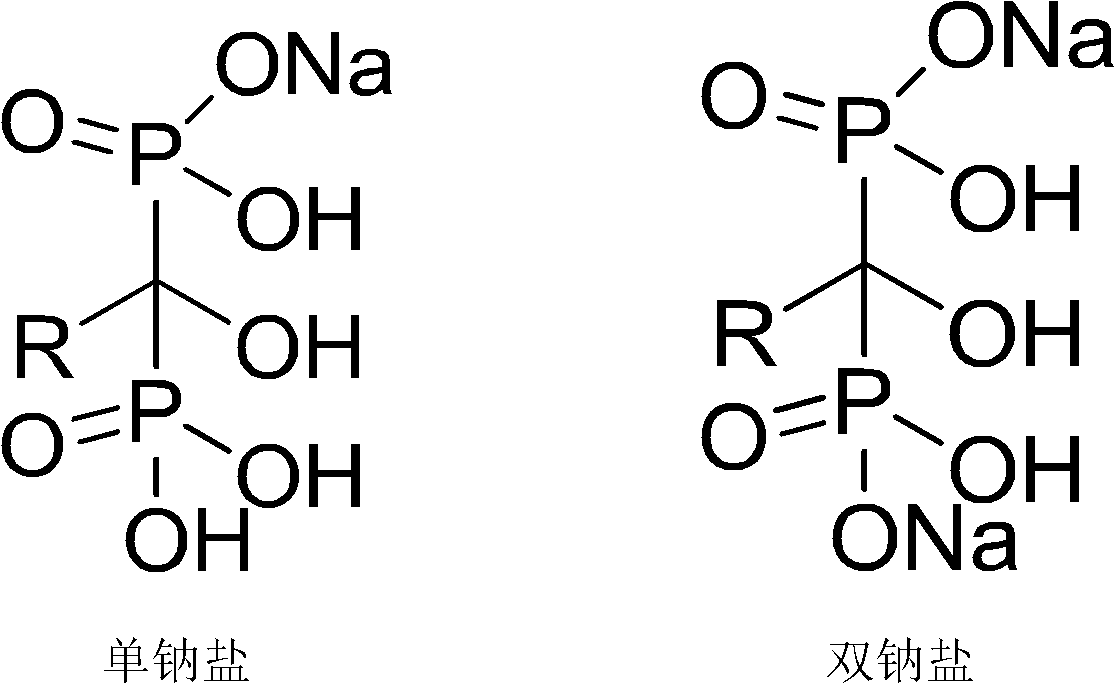
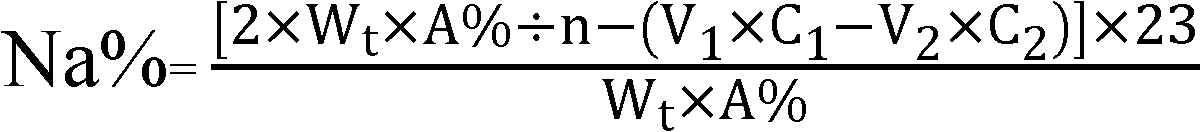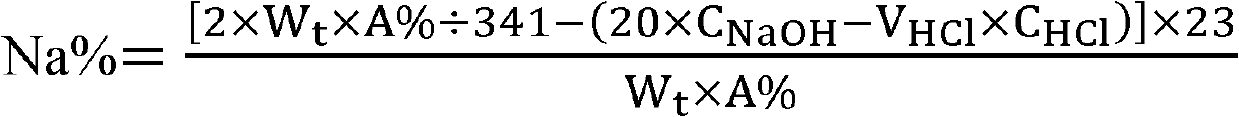Method for determining sodion content in diphosphonic acid monosodium salt compound
A monosodium salt and bisphosphonic acid technology, which is applied in the field of physical and chemical analysis of chemical substances, can solve the problems of lack of sodium ion content determination methods, high detection environment requirements, and strict processing conditions, so as to make up for uncertainties and ensure product quality. High-quality, high-sensitivity results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] 1) Prepare the test solution: accurately weigh 250.0 mg of ibandronate sodium sample to be tested with an analytical balance, accurately add 10 ml of 0.5 mol / L sodium hydroxide titration solution, stir to dissolve the sample, and use it as the test solution;
[0065] 2) Titration reaction: add 0.5mol / L hydrochloric acid titration solution to the test solution prepared in step 1 and carry out neutralization titration;
[0066] 3) End point judgment: use the potentiometric method to judge the titration end point;
[0067] 4) content determination of sodium ion: according to the hydrochloric acid titrant volume of sample content and consumption, calculate the content of sodium ion in the ibandronate sodium sample to be measured according to the formula shown in formula I;
[0068] Repeat steps 1-4 twice, the three measured values are 6.62%, 6.58%, 6.67%, respectively, the average content of sodium ions in the sample is 6.62%, and the relative average deviation dr is 0.47...
Embodiment 2
[0070] 1) Prepare the test solution: accurately weigh 235.6 mg of ibandronate sodium sample to be tested with an analytical balance, accurately add 10 ml of 0.5 mol / L sodium hydroxide titration solution, stir to dissolve the sample, and use it as the test solution;
[0071] 2) Titration reaction: Add 3 drops of phenolphthalein indicator solution, add 0.5mol / L sulfuric acid titration solution to the test solution prepared in step 1 for neutralization titration;
[0072] 3) End point judgment: until the pink color disappears;
[0073] 4) content determination of sodium ion: according to the sulfuric acid titration solution volume of sample content and consumption, calculate the content of sodium ion in the ibandronate sodium sample to be measured according to the formula shown in formula I.
[0074] Repeat steps 1-4 twice, the three measured values are 6.67%, 6.63%, 6.65%, the average content of sodium ions in the sample is 6.65%, and the relative average deviation dr is 0.20%...
Embodiment 3
[0076] 1) Prepare the test solution: accurately weigh 201.3 mg of ibandronate sodium sample to be tested with an analytical balance, accurately add 20 ml of 0.1 mol / L sodium hydroxide titration solution, stir to dissolve the sample, and use it as the test solution;
[0077] 2) Titration reaction: add 3 drops of phenolphthalein indicator solution, add 0.2mol / L hydrochloric acid titration solution to the test solution prepared in step 1 for neutralization titration;
[0078] 3) End point judgment: until the pink color disappears;
[0079] 4) content determination of sodium ion: according to the hydrochloric acid titrant volume of sample content and consumption, calculate the content of sodium ion in the sodium ibandronate sample to be measured according to the formula shown in formula I.
[0080] Repeat steps 1-4 twice, the three measured values are 6.61%, 6.66%, 6.65%, the average content of sodium ions in the sample is 6.64%, and the relative average deviation dr is 0.30%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com