Method for greatly improving electrochemical performance of low-temperature hydrothermal synthesized LiFePO4
A hydrothermal synthesis and large-scale technology, applied in chemical instruments and methods, inorganic chemistry, phosphorus compounds, etc., can solve the problems of low yield per unit volume and high price of surfactants, achieve excellent electrochemical performance, increase yield, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Nanorod-shaped LiFePO was prepared by changing the order of precursor addition by using water as the reaction medium by the hydrothermal method 4 . In this embodiment, the iron salt is ferrous sulfate heptahydrate, the antioxidant is ascorbic acid, and the lithium salt is lithium hydroxide monohydrate.
[0038] Many problems faced by traditional methods are further solved only by changing the order of addition to prepare precursors. The method is: select 6.69g of lithium salt and dissolve it in 60mL of water. After the dissolution is complete, 4 mL of phosphoric acid (85wt.%) is added to it, and Li 3 PO 4 Suspension, where Li 3 PO 4 It is white precipitated nano-agglomerated particles. After the reaction was completed, 0.225 g of ascorbic acid and 14.6 g of iron salt were added thereto to finally form a blue slurry. The blue slurry was quickly transferred to a sealed reaction kettle, and hydrothermally reacted at 180° C. for 3 hours. Then separated to...
Embodiment 2
[0040] Example 2: Rod-shaped LiFePO prepared by changing the order of precursor addition and adding a certain amount of ethylene glycol 4 . The iron salt adopts ferrous sulfate heptahydrate, the antioxidant adopts ascorbic acid, and the lithium salt adopts lithium hydroxide monohydrate.
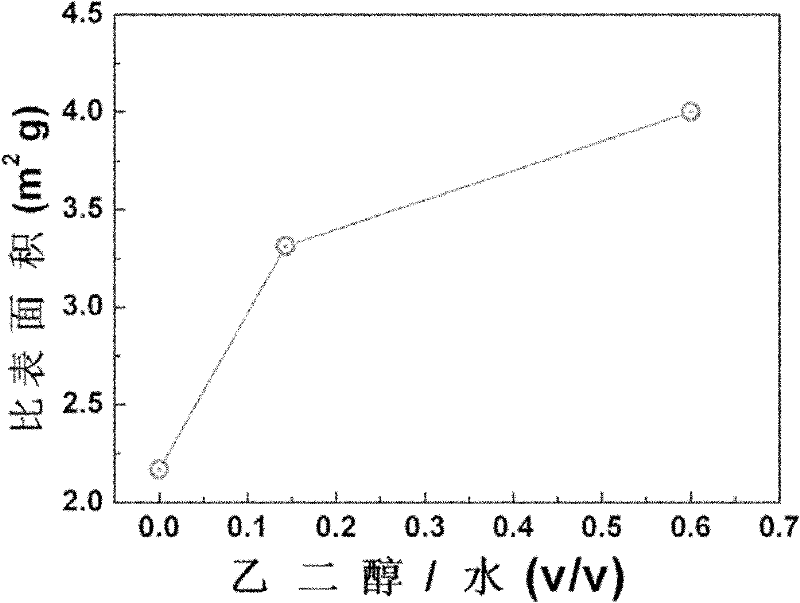
[0041] The method is: select 6.69g of lithium salt and dissolve it in 30mL of water so that the concentration of the lithium salt solution is 0.223gmL -1 . After the dissolution is complete, 4 mL of phosphoric acid (85wt.%) is added to it, and Li 3 PO 4 Suspension, where Li 3 PO 4 It is white precipitated nano-agglomerated particles. After the reaction was completed, 0.225 g of ascorbic acid and 14.6 g of iron salt were added thereto to finally form a blue slurry. Subsequently, a certain amount of ethylene glycol solution 60mL was added thereto, and after being uniformly mixed, it was quickly transferred to a sealed reaction kettle, and hydrothermally reacted at 180° C. for 3 hours. T...
Embodiment 3
[0043] Embodiment 3: the difference from embodiment 2 is that the synthesis temperature and reaction time used in the experiment are different. The prepared rod-shaped LiFePO 4 , with a diameter of 50-80nm and an aspect ratio of 1-8. In this embodiment, the iron salt is ferrous sulfate heptahydrate, the antioxidant is ascorbic acid, and the lithium salt is lithium hydroxide monohydrate.
[0044] The volume ratio of ethylene glycol: water in the ethylene glycol solution is 1:1. The hydrothermal conditions are: temperature 150° C., time 10 hours.
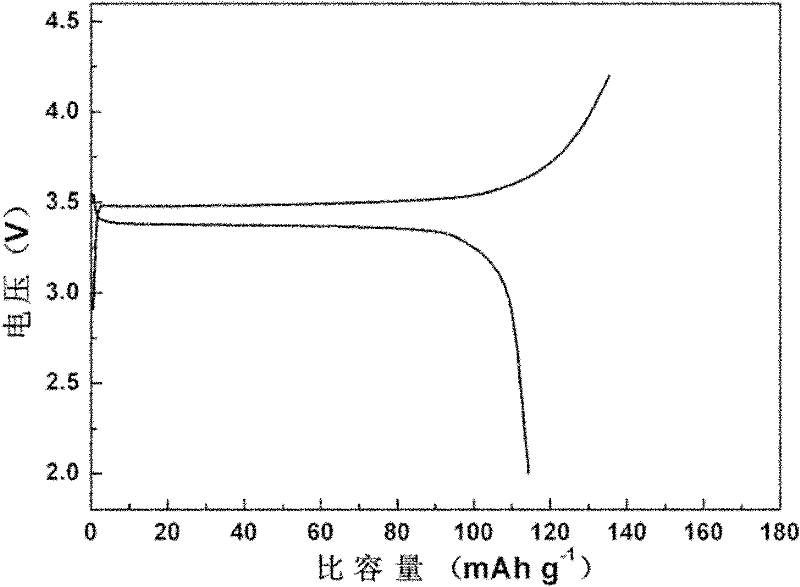
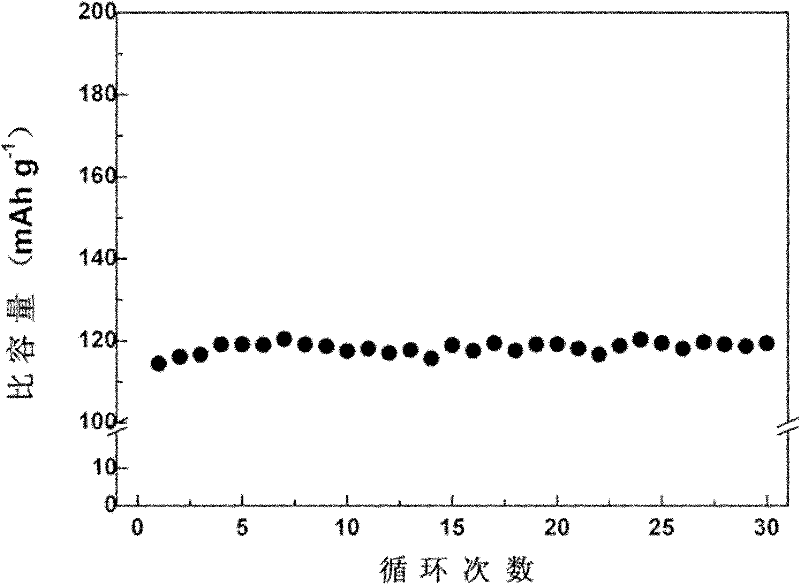
[0045] The obtained product is LiFePO 4 Simplex. The first discharge specific capacity at 0.1C charge and discharge rate reached 160mAh g -1 , and good cycle performance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com