Immune adjuvant of foot-and-mouth disease vaccine and application of immune adjuvant
A technology for foot-and-mouth disease vaccine and immune adjuvant is applied in the field of vaccine adjuvant and its application, preparation of immune adjuvant for foot-and-mouth disease vaccine, and can solve the problems of delayed onset time, undetectable and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of the different fragments of embodiment 1 foot-and-mouth disease virus 3D protein
[0032] 1. Bioinformatics analysis of 3D protein gene of foot-and-mouth disease virus:
[0033] The non-structural protein 3D of foot-and-mouth disease virus is a viral RNA polymerase, which is highly conserved in seven serotypes of foot-and-mouth disease virus. The full length of the 3D gene is 1410 nucleotides, encoding a protein with a length of 470 amino acids. DNAStar biological software was used to analyze the sequences of 46 representative strains of seven serotypes and compare them with the GenBank database. The results showed that the nucleotide The homology is higher than 90%, and the amino acid homology is higher than 97%, highly conservative. In this study, the 3D sequence of Asia type 1 FMD virus JS / China / 05 strain was used as a reference sequence to design primers to amplify the target gene fragment and express the protein.
Embodiment 2
[0036] The preparation of embodiment 2 recombinant antigen (EoIgG)
[0037] 1. Bioinformatics analysis of O-type foot-and-mouth disease virus VP1 gene:
[0038] The structural protein VP1 of foot-and-mouth disease virus is the dominant antigen of the virus. Whether it is the isolated and purified natural VP1 protein or the recombinant expression product, it can induce the body to produce protective neutralizing antibodies, which is type-specific. The full length of the foot-and-mouth disease virus VP1 gene consists of 639 nucleotides, encoding a protein with 213 amino acids, and its main epitopes are concentrated in the 140-160 amino acid and the 200-213 amino acid segment. The present invention uses DNAStar biological software to carry out sequence analysis to three strains of porcine foot-and-mouth disease virus O type representative strains (O / China / 99, O / Miandian / 98, O / ZK / 93) that China isolates, and determined that the dominant antigenic epitope is The 135-160th amino ac...
Embodiment 3
[0046] Embodiment 3, vaccine preparation and immune efficacy experiment:
[0047] 1. Vaccine preparation
[0048] The purified recombinant antigen prepared in Example 2 and the different fragments (3D1\3D2\3D3) of the purified 3D protein prepared in Example 1 were quantified by the Bio-Rad quantitative kit and then diluted to an appropriate concentration, purified After the 3D protein fragments and recombinant antigens were configured in a ratio of 1:2 (V / V), an equal volume of oil adjuvant ISA206 (France) was added to emulsify into a vaccine preparation, which was packed in 1ml / tube, which contained 200 μg of recombinant antigen, 3D Protein fragments 100 μg.
[0049] 2. Immunity efficacy test:
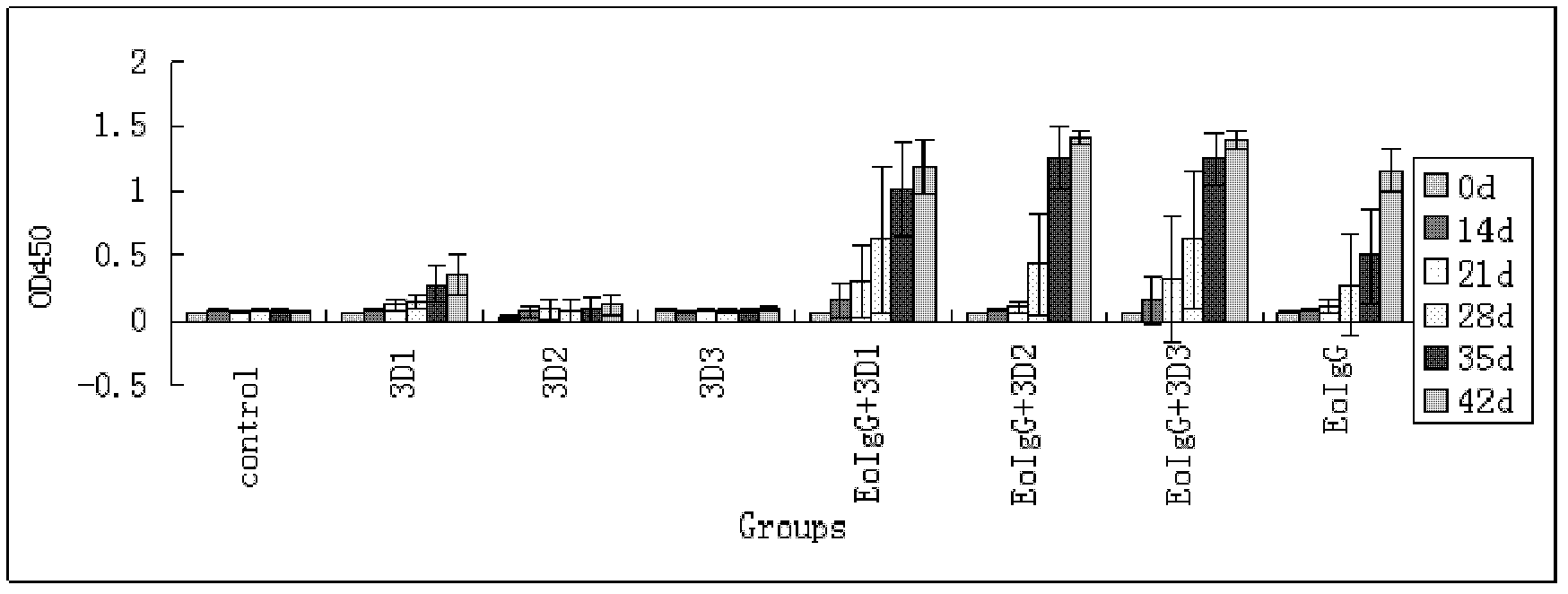
[0050] Use the prepared vaccine to inoculate guinea pigs (100 μg antigen + 50 μg 3D protein fragments) by intramuscular route with 0.5 ml per head and inoculate the animal pigs (200 μg antigen + 100 μg 3D protein fragments) with 1 ml per head for immunization The experimental resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com