Novel method for preparing dihydroxyurea
A technology of dihydroxyurea and hydroxylamine, applied in the field of preparing dihydroxyurea, which can solve the problems of low yield of dihydroxyurea, incomplete dissolution, inability to dissociate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
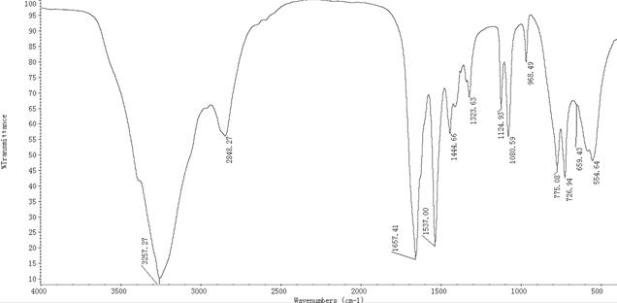
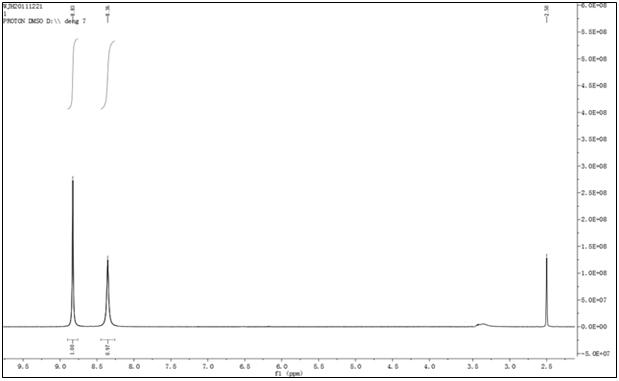
Image
Examples
Embodiment
[0014] Example: The present invention uses hydroxylamine and solid phosgene to prepare under anhydrous conditions, so all reaction vessels are dried and kept dry. and carried out under dry experimental or synthetic conditions.
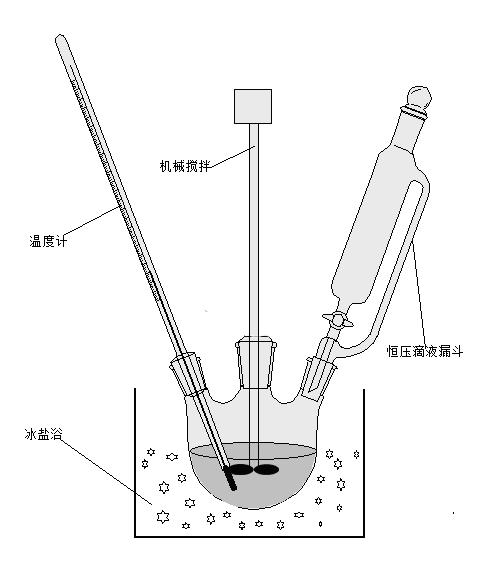
[0015] The preparation process is as follows: 1. First, add 0.3mol hydroxylamine and a certain amount of potassium acetate into a three-necked flask ( figure 1 is the overall reaction device), put the three-necked flask in an ice-salt bath to ensure that the reaction conditions are below 0°C (ie, freezing point), and immediately start the mixer until the mixture becomes an emulsion (called A solution).
[0016] 2. In addition, a certain amount of solid phosgene-that is, bis(trichloromethyl)carbonate (Cl 3 COCOOCCl 3 ), dissolved in 1,4-dioxane, and then poured into the constant pressure titration funnel, carefully placed the constant pressure titration funnel on the above three-necked flask, and placed the thermometer in the three-necked flask (bot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com