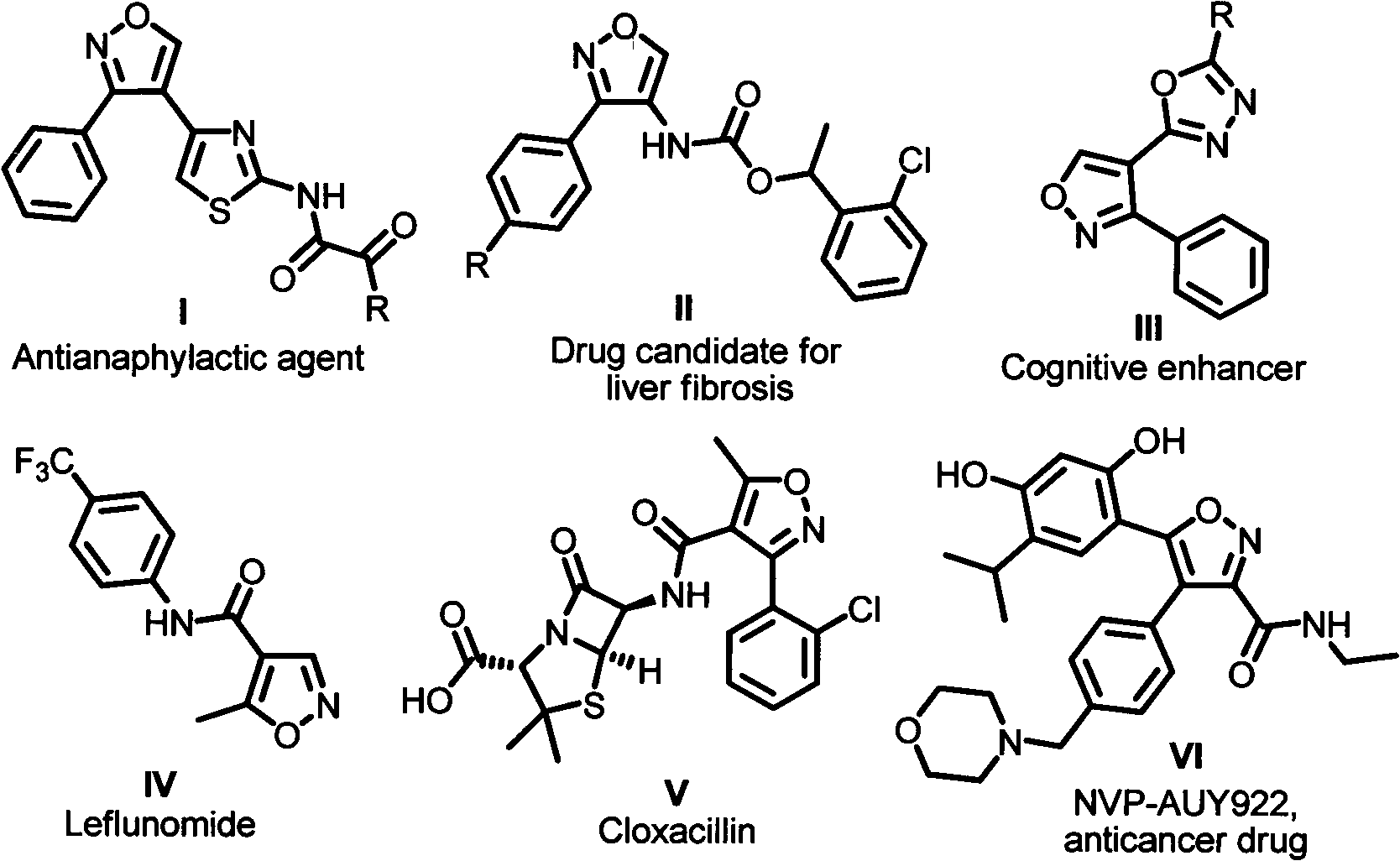
Synthetic method of 3,4-disubstituted isoxazole compound
A synthesis method and technology for aldehyde compounds, applied in the field of organic compound process application, can solve the problems of low yield and the like, and achieve the effects of high efficiency, anti-allergic activity, anti-tumor activity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthetic method of substrate 1:
[0026]
[0027] Substrate A (10.0 mmol) was dissolved in 10 mL of ethanol and 0.5 mL of pyridine, and heated to 80°C. After stirring for 5 minutes, hydroxylamine hydrochloride (12.0 mmol) was added in one portion. React at 80° C. for 2 hours, detect the reaction on a TLC plate, and spin off the solvent after substrate A disappears. Add 5 mL of DCM, stir at 0oC for 10 minutes, and a suspension appears. The solid was filtered off, and the filtrate was spin-dried to obtain product B. The product B (8.75 mmol) was dissolved in 10 mL of DCM, 5 drops of pyridine was added dropwise, and the mixture was lowered to 0°C and stirred for 5 minutes. Then, NCS (10.5 mmol) was added in portions over 30 minutes. After the addition was complete, the reaction was warmed to room temperature and the reaction was stirred until B disappeared. 20 mL of water was added and extracted with DCM, and the yellow powder product 1 (N-hydroxychloroimide c...
Embodiment 2
[0032]
[0033] In a reaction flask, dissolve substrate 3a (74.5 μL, 0.88 mmol) and TEA (53.9 μtL, 0.4 mmol) in 4 mL of toluene, and after adjusting the temperature to 0 ° C, add substrate 2a (173.9 μL, 1.6 mmol) into the reaction. Then, the substrate 1a (62.2mg, 0.4mmol) was dissolved in 0.2mL toluene, and added to the reaction system in 5 times. After the reaction was carried out at 0°C for 10 minutes, the temperature was adjusted to room temperature, and the reaction was carried out for 1.5 hours. TLC tracking point plate, after the end of the reaction, flash column chromatography purification to obtain product 4aa (100mg, 98%). 1 H NMR (300MHz; CDCl 3): δ=7.75-7.69(m, 2H), 7.48-7.41(m, 3H), 5.41(d, J=2.7Hz, 1H), 3.40(dd, J=3.5, 2.8Hz, 1H), 2.91- 2.68(m, 4H), 2.22-2.13(m, 1H), 1.83-1.78(m, 4H), 1.10(d, J=6.9Hz, 3H), 0.83(d, J=6.9Hz, 3H); 13 C NMR (75MHz, CDCl 3 ): δ=157.2, 129.6, 128.8, 126.8, 95.3, 56.2, 46.9, 28.1, 23.8, 20.6, 17.1; HRMS (ESI) calcd for C 16 h 2...
Embodiment 3
[0035]
[0036] The operation was the same as in Example 2, and THF was used as the solvent, and the yield was 93%. The product was detected as compound 4aa by spectrogram.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com