Aliphatic polyester grafted polyamino acid copolymer and preparation method thereof
An aliphatic polyester, polyamino acid technology, applied in the direction of medical science, prosthesis, etc., can solve the problems of insufficient mechanical properties of polyamino acid, limited application, etc., and achieve the effect of good biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
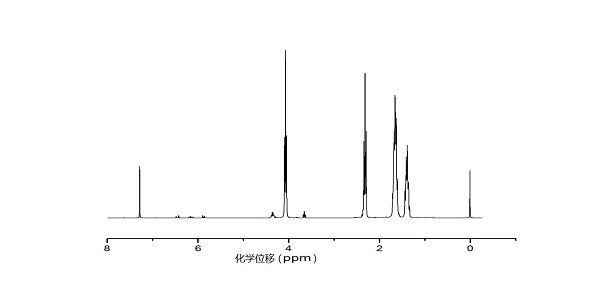
[0031] Example 1: Synthesis of poly(ε-caprolactone)
[0032] Weigh 3 parts of 11.4g (0.1mol) of ε-caprolactone into an ampoule, add 57mL of toluene respectively, then add 1mL of 0.1mol / L stannous octoate in toluene, then add 10mL, 5mL, 0.4 mL of 1 mol / L toluene solution of isopropanol was placed in an oil bath at 120°C for 24 hours, and then precipitated with 570 mL of ether, and the resulting product was vacuum-dried for 24 hours to obtain poly(ε-hexyl) with different number average molecular weights. ester).
[0033] Table 1 The number average molecular weight and reaction yield of the obtained polyε-caprolactone
[0034] sample name Theoretical Degree of Polymerization (DP) Number average molecular weight Mn Reaction yield (%) Poly(ε-caprolactone) 10 10 1085 93.7 Poly(ε-caprolactone) 20 20 2111 95.1 Poly(ε-caprolactone) 250 250 27191 95.6
[0035] In the above table, the number average molecular weight Mn is the number aver...
Embodiment 2
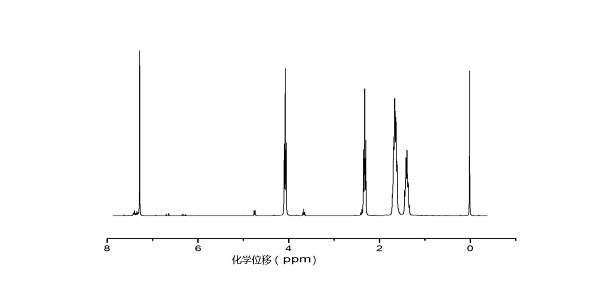
[0036] Example 2: Synthesis of polylactic acid
[0037] Weigh 3 parts of 7.2 g lactide into ampoules, add 36 mL of toluene solution, then add 1 mL of 0.1 mol / L stannous octoate in toluene, then add 6.67 mL, 2 mL, and 0.33 mL of 1 mol / L L of toluene solution of isopropanol was placed in an oil bath at 120°C for reaction. After 24 hours, it was precipitated with 360 mL of ether, and the resulting product was vacuum-dried for 24 hours to obtain polylactic acid.
[0038] Table 2 The number average molecular weight and reaction yield of polylactic acid obtained
[0039] sample name Theoretical Degree of Polymerization (DP) Number average molecular weight Mn Reaction yield (%) polylactic acid 15 15 1067 91.2 polylactic acid 50 50 3443 92.1 polylactic acid 300 300 20867 92.7
[0040] In the above table, the number average molecular weight Mn is the number average molecular weight of the reaction product polylactic acid, by 1 H NMR me...
Embodiment 3
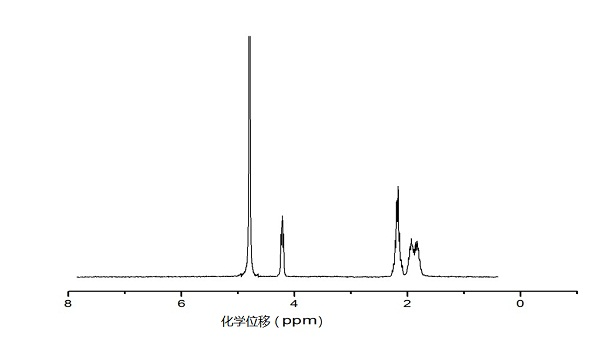
[0041] Example 3: Synthesis of Hydroxyethyl Acrylate-Poly(ε-caprolactone)
[0042] Weigh three parts of 11.4g (0.1mol) of ε-caprolactone into the ampoule, add 57mL of toluene respectively, then add 1mL of 0.1mol / L stannous octoate in toluene, then add 10mL, 5mL, 0.4 mL of 1 mol / L hydroxyethyl acrylate toluene solution was placed in an oil bath at 120°C for reaction, and after 24 hours, it was precipitated with 570 mL of ether, and the resulting product was vacuum-dried for 24 hours to obtain hydroxyethyl acrylate-poly(ε-hexyl lactone).
[0043] Table 3 The number average molecular weight and reaction yield of the obtained hydroxyethyl acrylate-poly(ε-caprolactone)
[0044] sample name Theoretical Degree of Polymerization (DP) Number average molecular weight Mn Reaction yield (%) Poly(ε-caprolactone) 10 10 1255 92.1 Poly(ε-caprolactone) 20 20 2281 93.4 Poly(ε-caprolactone) 250 250 27817 94.7
[0045] In the above table, the num...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com