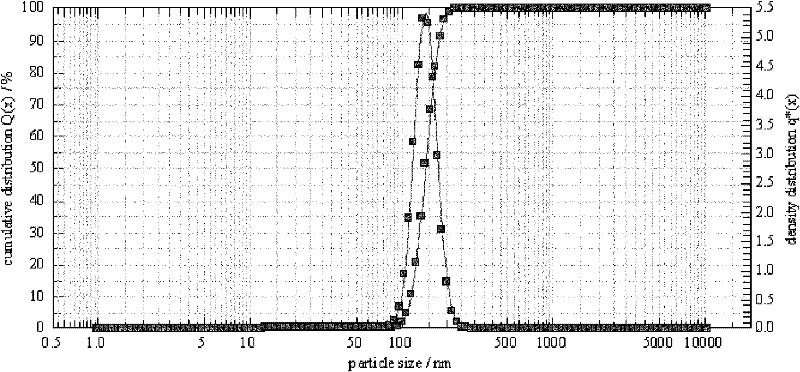
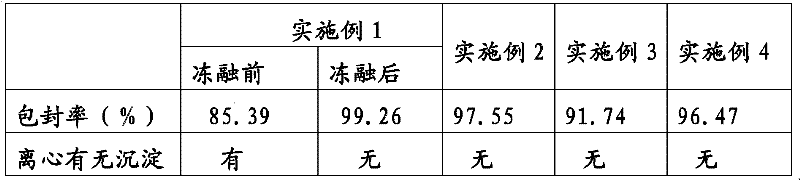
Lipid preparation containing insoluble camptothecin drug and preparation method thereof
A technology of liposome preparations and camptothecin, which is applied in the field of pharmaceutical preparations, can solve the problems of low liposome encapsulation efficiency and poor affinity, and achieve the effect of improving encapsulation efficiency, simple preparation method, and improving encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 110
[0037] The preparation of embodiment 110-hydroxycamptothecin long circulation thermosensitive liposome
[0038] Prepare liposomes by film dispersion-homogeneous membrane-passing method: 10-hydroxycamptothecin 1g, DPPC (dipalmitoyl lecithin) 46g, distearylethanolamine-polyethylene glycol 2000 3.0g, dissolve in chloroform : Methanol = 1:1, sonicate until completely dissolved, 55°C, 150rpm rotary evaporation for 40min to remove the organic solvent, vacuum-dry for 3h, add 0.3M, pH5.0 sodium phosphate salt solution containing 500mg / ml trehalose After hydration at 50°C and 100rpm for 40 minutes, homogenize through a 0.15 μm polycarbonate membrane, and then divide the mixture after passing through a 0.22 μm membrane into two parts, one for direct storage and use, and the other for freeze-thawing as follows After storage and use, the long-circulation thermosensitive liposome of 10-hydroxycamptothecin can be obtained.
[0039] Freeze-thaw procedure:
[0040] Freeze-Thaw Program -70°C...
Embodiment 210
[0042] The preparation of embodiment 210-hydroxycamptothecin liposomes
[0043] Dissolve 2 g of 10-hydroxycamptothecin, 47.5 g of egg yolk lecithin, and 0.5 g of vitamin E in chloroform:methanol=2:1, sonicate until completely dissolved, and rotatively evaporate at 45°C and 150 rpm for 40 minutes to remove the organic solvent, vacuum Dry for 3 hours, add 0.5M sodium phosphate solution with pH 5.0 containing 400mg / ml sucrose, hydrate at 45°C and 100rpm for 40min, homogenize through a 0.10μm polycarbonate membrane, and store for use after repeated freezing and thawing , to obtain 10-hydroxycamptothecin liposomes.
Embodiment 39
[0044] The preparation of embodiment 39-aminocamptothecin long circulation liposome
[0045] Dissolve 4g of 9-aminocamptothecin, 43g of egg yolk lecithin, 2.5g of distearylethanolamine-polyethylene glycol, and 0.5g of cholesterol in chloroform:methanol=2:1, sonicate until completely dissolved, 50°C, 150rpm Rotary evaporation at a rotating speed of 40 min to remove the organic solvent, vacuum drying for 3 h, adding 0.7 M sodium phosphate solution containing 300 mg / ml sucrose, pH 5.0, hydration at 45 °C and 100 rpm for 40 min, and homogenizing through 0.10 μm poly The carbonate membrane is stored and used after repeated freezing and thawing to obtain 9-aminocamptothecin long-circulating liposomes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com