Method for recycling ammonia and nitrogen in waste watery by aid of chemicrystallization
A chemical crystallization and wastewater technology, which is applied in chemical instruments and methods, water/sewage treatment, water/sludge/sewage treatment, etc., can solve the problems of high cost of chemicals and decreased removal rate of ammonia nitrogen, so as to reduce the cost of chemicals and improve the Release rate, the effect of solving the high cost of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The coking wastewater was taken, and the quality of the wastewater is shown in Table 1.
[0034] Table 1 Water quality of coking wastewater
[0035] parameter unit concentration COD mg / L 220±10 pH - 7.2±0.1 NH 4 -N mg / L 650±20 BOD mg / L 50±1 Ca 2+ mg / L 21±0.5
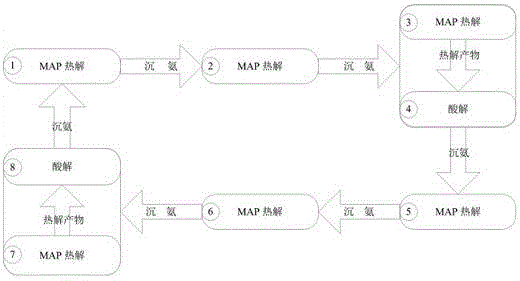
[0036] A kind of chemical crystallization method of the present embodiment reclaims the method for ammonia nitrogen in waste water, and its steps are:
[0037] (1) Prefabrication of magnesium ammonium phosphate crystals, adding sodium hydroxide powder to the solid ammonium magnesium phosphate for pyrolysis, the mass ratio of sodium hydroxide: magnesium ammonium phosphate is 40:245, the pyrolysis temperature is 110 ℃, and the pyrolysis time is 3 h, the ammonia produced by pyrolysis is recovered by a sulfuric acid solution with a mass fraction of 2%;
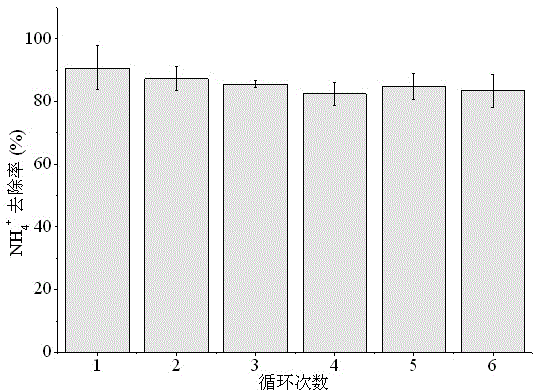
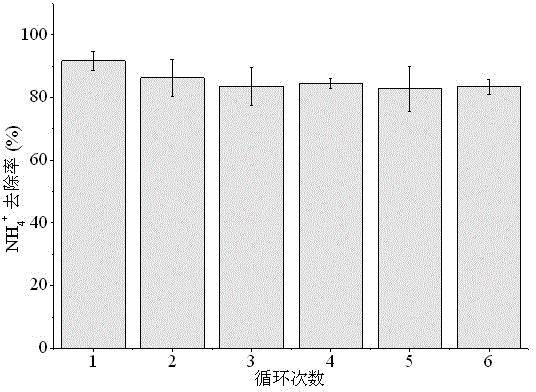
[0038] (2) Put the pyrolysis product prepared in step (1) into the waste water in Table 1, wherein, the m...
Embodiment 2
[0044] Breeding wastewater was taken, and the quality of the wastewater is shown in Table 2.
[0045] Table 2 Water quality of aquaculture wastewater
[0046] parameter unit concentration COD mg / L 550±10 pH - 7.4±0.1 NH 4 -N mg / L 860±20 BOD mg / L 90±10 TOC mg / L 120±10
[0047] A kind of chemical crystallization method of the present embodiment reclaims the method for ammonia nitrogen in waste water, and its steps are:
[0048] (1) Prefabrication of magnesium ammonium phosphate crystals, adding sodium hydroxide powder to the solid ammonium magnesium phosphate, the mass ratio of sodium hydroxide: magnesium ammonium phosphate is 80:245, the pyrolysis temperature is 100 °C, and the pyrolysis time is 2 h. Ammonia produced by pyrolysis is recovered with a sulfuric acid solution with a mass fraction of 2%;
[0049] (2) Add the pyrolysis product obtained in step (1) to the breeding wastewater in Table 2. The mass ratio of pyro...
Embodiment 3
[0055] The waste water from the fertilizer industry was taken, and the quality of the waste water is shown in Table 1.
[0056] Table 3 Wastewater quality of chemical fertilizer industry
[0057] parameter unit concentration COD mg / L 560±10 pH - 7.86±0.1 NH 4 -N mg / L 1197±20 SS mg / L 10±1 Mg 2+ mg / L 0.516±0.1
[0058] A kind of chemical crystallization method of the present embodiment reclaims the method for ammonia nitrogen in waste water, and its steps are:
[0059] (1) Prefabrication of magnesium ammonium phosphate crystals, adding sodium hydroxide powder to the solid ammonium magnesium phosphate, the mass ratio of sodium hydroxide: magnesium ammonium phosphate is 60:245, the pyrolysis temperature is 105 °C, and the pyrolysis time is 2.5 h. Ammonia produced by pyrolysis is recovered with a sulfuric acid solution with a mass fraction of 2%;
[0060] (2) Put the pyrolysis product obtained in step (1) into the waste w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com