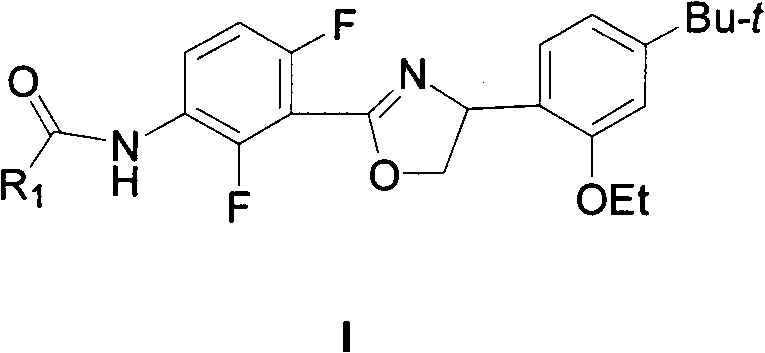
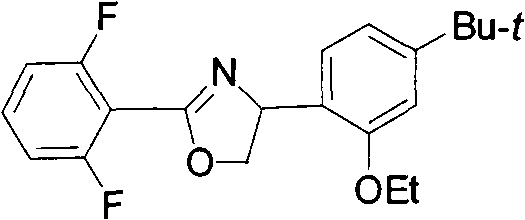
New 2,4-diphenyloxazoline compounds, and synthesis method and acaricidal activity thereof
A technology of diphenyloxazoline and compound is applied in the field of 2,4-diphenyloxazoline new compound, synthesis and its acaricidal activity, and can solve problems such as poor quick effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Synthesis of m-tert-butylanisole (I-1)
[0040] Add 30 g of m-chloro-tert-butylphenol, 160 g (10%) NaOH and 150 mL of benzene into a 500 mL four-neck flask, heat to 70° C., and continue stirring for 1 h. After cooling to 65°C, 22.8 g of dimethyl sulfate was added dropwise. The dropwise addition was completed within 1 hour, and the temperature was kept for 1.5 hours to 4 hours, followed by TLC until the end of the reaction. Cool to 20°C, let stand, separate the organic layer, extract the water layer with benzene (30mL×2), combine the organic layers, and remove the solvent by rotary evaporation. Distilled at 68-72° C. / 267 Pa, 33.3 g of a light yellow transparent liquid was obtained, and the yield was 92%.
[0041] (2) Synthesis of 3-nitro-2,6-difluorobenzoic acid (I-2)
[0042] (1) Preparation of mixed acid: slowly add concentrated sulfuric acid (120 g) into concentrated nitric acid (53 g) under the condition of an ice-water bath. Mix well and cool for later use. ...
Embodiment 2
[0055] Example 2: 2,3-difluoro-N-[3-((4-(2-ethoxy-4-tert-butyl)-phenyl)-4,5-dihydro-1,3-oxa Synthesis of azole-2-yl)-2,4-difluorophenyl]-pyridine-4-carboxamide (2a)
[0056] Add 1.87g of compound I-7 (0.6mmol), 1.26g of triethylamine and 20mL of dry tetrahydrofuran (THF) into a 100mL four-necked flask, and cool to 0°C. Dissolve 1.17g of 2,3-difluoropyridine-4-carbonyl chloride (0.66mmol) in 3mL of tetrahydrofuran, and slowly drop it into a four-necked flask. After the dropwise addition was completed, stir for 3 h, then return to room temperature, and track the reaction by TLC. After the reaction, the reaction liquid was desolvated and separated by column chromatography (silica gel 200-300 mesh), the yield: 65%. 1 H NMR (300MHz, CDCl 3 )δ10.77(s, 1H), 8.19(dd, J=5.0, 1.4Hz, 1H), 7.99(td, J=9.0, 5.8Hz, 1H), 7.69(t, J=4.9Hz, 1H), 7.35(td, J=9.2, 1.6Hz, 1H), 7.20(d, J=8.0, 1H), 6.97(m, 2H), 5.53(dd, J=10.4, 8.4Hz, 1H), 4.84(dd, J=10.4, 8.4Hz, 1H), 4.13(m, 3H), 1.34(t, J=6.9Hz...
Embodiment 3
[0057] Example 3: 2,4-difluoro-N-[3-((4-(2-ethoxy-4-tert-butyl)-phenyl)-4,5-dihydro-1,3-oxa Synthesis of azole-2-yl)-2,4-difluorophenyl]-pyridine-3-carboxamide (2b)
[0058] Add 1.87g of compound I-7 (0.6mmol), 1.26g of triethylamine and 20mL of dry tetrahydrofuran (THF) into a 100mL four-necked flask, and cool to 0°C. 1.17g of 2,4-difluoropyridine-3-carbonyl chloride (0.66mmol) was dissolved in 3mL of tetrahydrofuran, and slowly dropped into a four-necked flask. After the dropwise addition was completed, stir for 3 h, then return to room temperature, and track the reaction by TLC. After the reaction, the reaction solution was desolvated and separated by column chromatography (silica gel 200-300 mesh), yield: 70%. 1 H NMR (400MHz, CDCl 3 )δ8.67(m, 1H), 8.30(m, 2H), 7.27(m, 1H), 6.97(m, 4H), 5.60(ddd, J=12.0, 10.4, 8.5Hz, 1H), 4.87(dt , J=10.5, 8.1Hz, 1H), 4.13(m, 4H), 1.43(t, J=6.9, 5.8Hz, 3H), 1.33(s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com