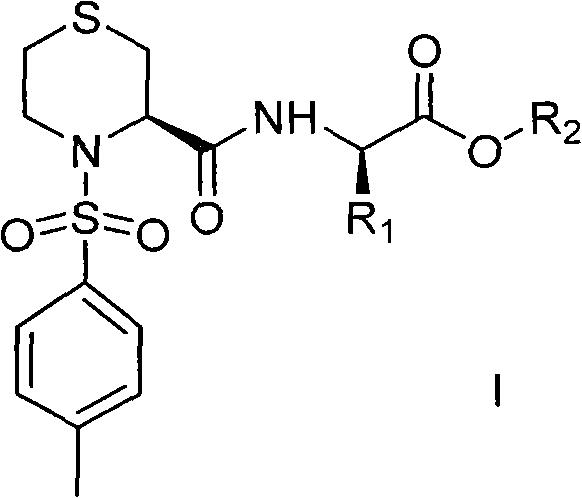
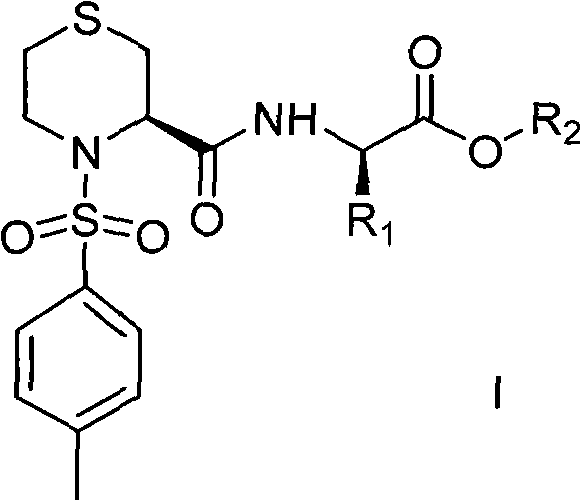
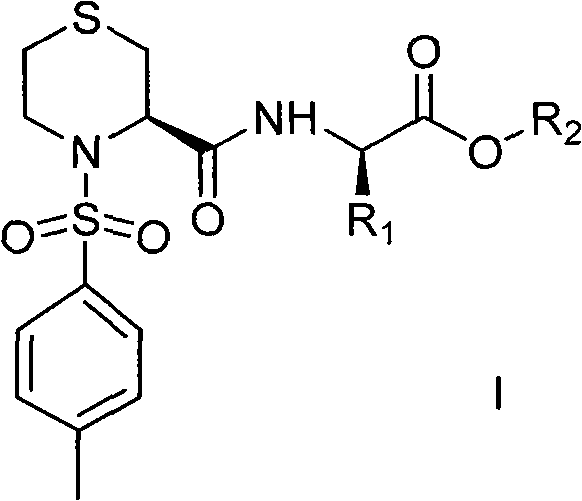
Thiazine amide derivative and application thereof in preparation of medicines for preventing and controlling neurodegenerative diseases
A technology of thiazinamide and derivatives, applied in the field of compounds of formula I, can solve the problems of unsuitability for prevention and treatment of neurodegenerative diseases, low melting point, poor blood-brain barrier passing ability, etc., and achieves excellent processability and good fluidity. , the effect of high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 2-Hydroxyethylcysteine
[0026] Add 109 g (0.9 mol) of cysteine to a 2000 ml round bottom flask, dissolve it with 1000 ml of distilled water, cool to 10°C in an ice bath, and add 24 ml of 1M NaOH aqueous solution to neutralize to pH-7. Pipette 100 ml of ethylene oxide cooled in advance at 10°C. After the addition, react at a constant temperature at 10°C for 1 hour, then rise to room temperature and react for 1.5 hours.
[0027] Extract with ether 400ml×4 to remove unreacted ethylene oxide. The water layer in the system was evaporated under the condition of less than 60℃ to obtain a yellow solid, which was recrystallized with water:ethanol=85ml:350ml, filtered and washed thoroughly with 95% ethanol to obtain the target compound, a white scaly solid, mp195 -196°C, about 100 grams, yield 67.5%. 1 H-NMR (400MHz, D 2 O)δ: 3.96131(dd, 1H, J 1 =4.272Hz, J 2 =7.816Hz), 3.80680-3.77293(m, 2H), 3.17887(dd, 1H, J 1 =4.268Hz, J 2 = 14.814Hz), 3.08224 (dd, 1H, J 1 =7.480Hz,...
Embodiment 2
[0028] Example 2 2-Chloroethylcysteine hydrochloride
[0029] Add 44 g of 2-hydroxyethylcysteine to a 1000 ml round bottom flask, dissolve it in 600 ml of concentrated hydrochloric acid, and heat to an external temperature of 90-95° C. and stir to react for 7 hours. After the reaction was completed, let it stand overnight in a refrigerator, and a large amount of needle-like solids precipitated in the system. The solvent was removed by suction filtration, and the obtained solid was naturally dried to obtain an off-white solid, mp185-186°C. About 40 grams, yield>70%. 1 H-NMR (400MHz, D 2 O) δ: 4.30477-4.26952 (m, 1H), 3.81913-3.78409 (m, 2H), 3.25903 (dd, 1H, J 1 =4.444Hz, J 2 = 14.984 Hz), 3.18877 (dd, 1H, J 1 = 7.352 Hz, J 2 =15.072 Hz), 3.04410-3.00625 (m, 2H).
Embodiment 3
[0030] Example 3 L-1,4-thiazine-3-carboxylic acid hydrochloride
[0031] Take 20 grams of 2-chloroethylcysteine hydrochloride, dissolve it in water, add 7.2 grams of NaHCO dropwise under ice bath 3 After the addition, stir thoroughly to neutralize, extract 3 times with ethyl acetate, combine the organic phases, and Na 2 SO 4 dry.
[0032] The solvent was evaporated under reduced pressure, 400 ml of anhydrous methanol was added, and the reaction was stirred at room temperature for 5 days. The solvent was distilled off under reduced pressure and recrystallized with methanol: ether to obtain about 6 g of a white solid. mp160-161°C. 1 H-NMR(400M Hz, DMSO-d 6 )δ: 10.0898 (brs, 2H), 4.4214 (dd, 1H, J 1 =3.52Hz, J 2 =8.56Hz), 3.7833 (s, 3H), 3.4986-3.4766 (m, 1H), 3.2246-3.0606 (m, 3H), 2.9897-2.9593 (m, 1H), 2.8763-2.8622 (m, 1H); MS ( FAB) m / z: 162.0 (M-35.5), 102.0, 74.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com