External magnetic force for targeted cell delivery with enhanced cell retention
A cell and magnetic technology, applied in the fields of magnetic fields generated by permanent magnets, magnetic therapy, and drugs, etc., can solve the problem of low cell retention rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0239] Example 1: Isolation of Cardiac-Derived Stem Cells from Cardiac Biopsy Samples
[0240] Isolation of pluripotent stem cells from a cardiac biopsy or other cardiac tissue can be accomplished by any known method, such as the multi-step method described in US Publication No. 2008 / 026792, which is incorporated herein by reference.
[0241] Using this method, cardiac tissue is first obtained by percutaneous intramyocardial biopsy or by sterile dissection of the heart. However, it should be understood that in other embodiments, the initial tissue may be obtained by other methods (eg, surgical samples, fresh cadaver tissue, etc.). In some embodiments, harvesting of fresh tissue is not necessary since stem cells that have been previously isolated and stored (eg, cryopreserved) are used. Once obtained, tissue samples were kept on ice in high potassium cardioplegia (containing 5% glucose, 68.6 mmol / L mannose, 12.5 meq potassium chloride, and 12.5 meq sodium bicarbonate with 10...
Embodiment 2
[0245] Example 2: Isolation of porcine heart-derived cells
[0246] Porcine CDCs were isolated and cultured according to Davis et al., (2009) P10S One 4(9):e7195. Briefly, porcine myocardium samples were collected from the right ventricular septum using a biopsy knife. Biopsy samples were kept on ice in high potassium cardioplegia (5% glucose, 68.6 mM mannose, 12.5 meq potassium chloride, and 12.5 meq sodium bicarbonate and 10 units / mL heparin) to preserve tissue viability during transport . Tissues are processed within 2 hours. Myocardium samples were then sliced to less than 1mm 3 Fragments were washed and partially digested with collagenase. These tissue fragments were used as cardiac grafts in fibronectin (20 μg / mL; Sigma) coated dishes in cardiac transplantation medium (CEM; Iscove's Modified Dulbecco's Medium (GIBCO), fetal bovine serum (10% ( Piglets only); HyC10ne, 10gan, UT), 100 units / mL penicillin G (GIBCO), 100 μg / mL streptomycin (GIBCO), 2 mmol / L L-glutam...
Embodiment 3
[0247] Example 3: Tagging CDC with SPIO
[0248] SPIO microsphere particles (0.9-pm diameter, Bangs Laboratories, IN) and CDCs (1x10 in 15ml medium 6 Cells) were cultured in T75 flasks at a ratio of microspheres to cells of 500:1. As discussed herein, other ratios of microspheres (or other labeled particles) to cells (e.g., 100:1; 250:1; 1000:1; 2000:1, 4000:1, etc.) may be used in some embodiments ). Cells were incubated at 37°C and 95% air / 5% CO 2 conditions to introduce SPIO into the cells.
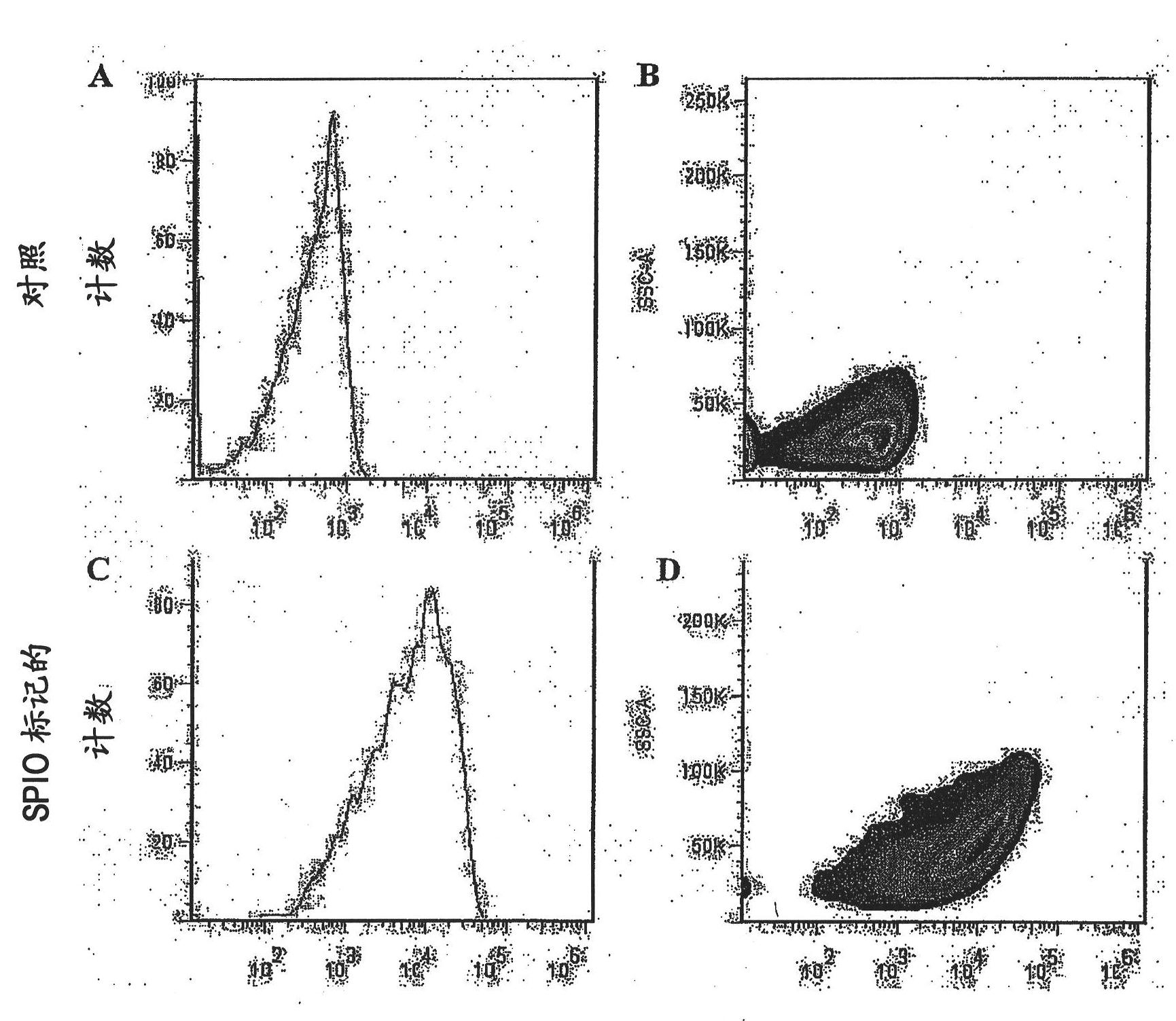
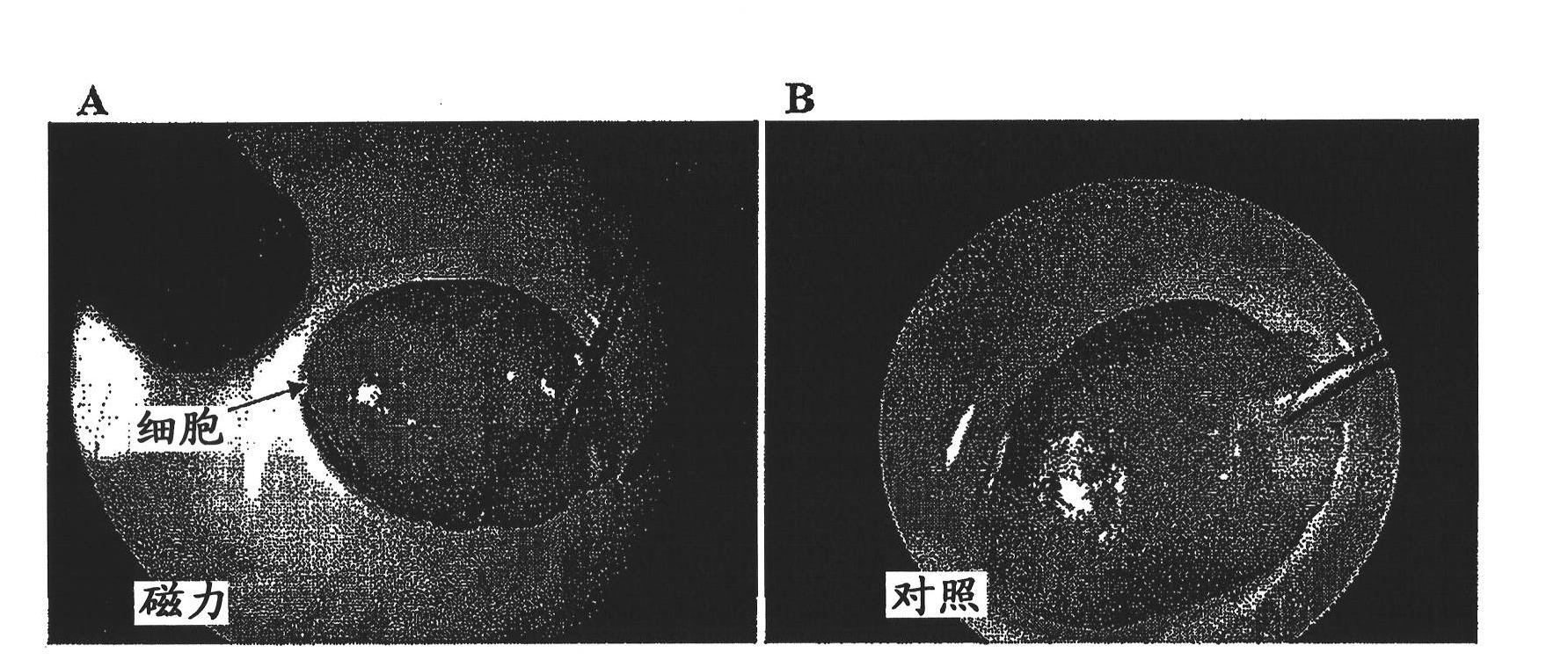
[0249] Labeling efficiency was evaluated by microscopy and flow cytometry. SPIO (with a dark green fluorescent label) overnight labeled CDC was examined with a fluorescence microscope (excitation 488 nm; emission 520 nm). A green color is observed, which indicates that the cells were successfully labeled by SPIO (see figure 1 ). Labeling efficiency was also analyzed by flow cytometry. As shown in Figure 2, when SPIO-labeled cells exhibited green fluorescence, the histograms w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com