Stem cell preparation for treating ischemic cardiomyopathy and preparation method of stem cell preparation
A technology of ischemic cardiomyopathy and stem cell preparation, applied in the field of stem cell biotechnology and cell transplantation, can solve the problems of limitations in stem cell growth and differentiation ability, difficulty in supplying stem cells, etc., achieve excellent heart repair ability, improve capillary Density, low immune rejection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Experimental conditions for preparing stem cells: In order to ensure the standardization and standardization of cell collection, separation, culture, and cryopreservation in vitro, to obtain cells that meet the clinical application level, and to ensure that cells can treat diseases in a safe and effective manner, the following guidelines should be followed: Relevant regulations at home and abroad, as well as the requirements of China's Good Manufacturing Practice (GMP), established a series of standard operating procedures (Standard Operating Procedure, SOP) for in vitro cell operations.
Embodiment 2
[0043] Example 2: The preparation method of placenta and umbilical cord stem cells, i.e. mesenchymal stem cells, is: the placenta and umbilical cord tissue from which blood stains have been removed are mechanically shredded into 1mm 3 The small pieces were infiltrated with collagenase Ⅰ with a concentration of 1mg / ml, digested at 37°C for 3 hours, and gently shaken once every 15 minutes; the cells were collected by filtering through a 100-mesh sieve; the cells were washed 3 times with D-Hank's balanced salt solution, Centrifuge at 200g for 8 minutes; resuspend the cells in high-glucose DMEM medium containing 10% fetal bovine serum, and adjust the cell density to 4.8×10 3 ~1×10 4 / cm 2 , inoculated in T75 culture flasks, as primary cells, at 37°C, with a volume fraction of 5% CO 2 Cultivate in the incubator, replenish the liquid after 48 hours, and change the liquid after 72 hours; when the cells grow to 80% confluent, digest with a mixture of 0.25% trypsin and 0.02% EDTA, ob...
Embodiment 3
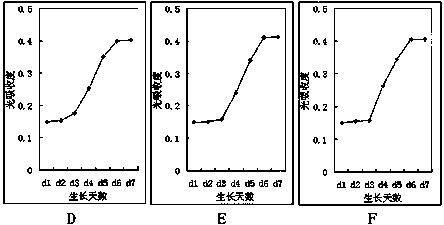
[0047] Example 3: The preparation method of myocardial stem cells is: take the third to fifth generation partial placenta and umbilical cord tissue stem cell suspension, and the cell concentration is 5×10 5 / dish was inoculated in 100mm petri dish, and after 24 hours of adherence, an induction system was added, using a low-sugar DMEM culture system with 10% fetal bovine serum, which contained 10 μmol / L of 5-azacytidine, and each dish contained 8 ml of induction system. After 24 hours of induction, the induction system was absorbed, washed with D-Hank's balanced salt solution, and then replaced with 10% fetal bovine serum low-sugar DMEM culture line to continue culturing for 2 weeks. Then it was expanded for three generations and collected for use; the growth state of cardiac stem cells after successful induction was also long spindle cells, such as figure 1 Shown in B.
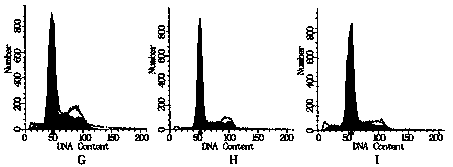
[0048] Determination of the growth activity of cardiac stem cells: collect cardiac stem cells induced and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com