Catalyst for alkylation of benzene and methanol, and preparation and application thereof
A technology of alkylation and catalyst, applied in the field of catalyst and its preparation and application, can solve rare problems and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
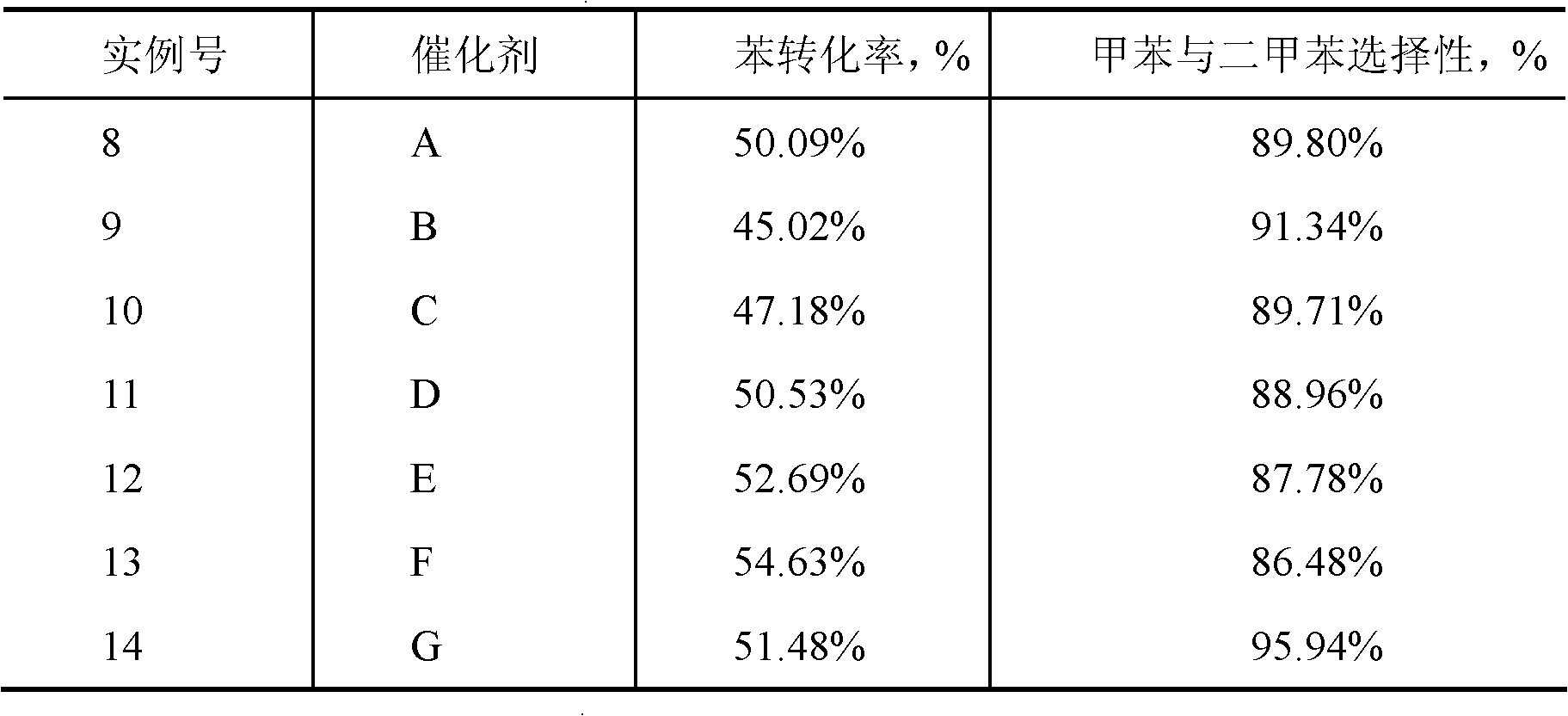
[0020] Take 15g of Na-type MCM-56 zeolite, and use 2N NH at 85±10°C 4 NO 3 The carrier was ion-exchanged with 300 mL of the solution for 6 hours, filtered, washed with 200 mL of deionized water, dried, and calcined at 540° C. for 6 hours to obtain H-type MCM-56 zeolite. Mix 10g of H-type MCM-56 zeolite with 4.286g of γ-Al 2 o 3 (Shanghai Petrochemical Research Institute) mixed, added 40mL of 4wt% nitric acid aqueous solution, kneaded, extruded, dried at 80°C for 12 hours, and roasted at 540°C in air for 6 hours to obtain a carrier. Analytical pure nickel nitrate [Ni(NO 3 ) 2 ·6H 2 O] 0.7008g and 10ml of water were made into an impregnating solution, and the above-mentioned 3g carrier was impregnated in an equal volume at room temperature for 24 hours, dried at 120°C for 12 hours, and roasted at 500°C in air for 6 hours to obtain the catalyst, and the obtained weight ratio was: NiO / Catalyst A of Type H MCM-56 / alumina = 6 / 70 / 30.
Embodiment 2
[0022] Take 15g of Na-type MCM-56 zeolite, and use 2N NH at 85±10°C 4 NO 3 The carrier was ion-exchanged with 300 mL of the solution for 6 hours, filtered, washed with 200 mL of deionized water, dried, and calcined at 540° C. for 6 hours to obtain H-type MCM-56 zeolite. Mix 10g of H-type MCM-56 zeolite with 4.286g of γ-Al 2 o 3 (Shanghai Petrochemical Research Institute) mixed, added 40mL of 4wt% nitric acid aqueous solution, kneaded, extruded, dried at 80°C for 12 hours, and roasted at 540°C in air for 6 hours to obtain a carrier. Analytical pure ammonium molybdate [(NH 4 ) 6 Mo 7 o 24 4H 2 O] 0.5520g, 10ml of water to make an impregnating solution, impregnate the above-mentioned 3g carrier in an equal volume at room temperature for 24 hours, dry at 120°C for 12 hours, and roast at 500°C in air for 6 hours to obtain the catalyst. The weight ratio obtained is: MoO 3 Catalyst B / Type H MCM-56 / Alumina = 15 / 70 / 30.
Embodiment 3
[0024] Take 15g of Na-type MCM-56 zeolite, and use 2N NH at 85±10°C 4 NO 3 The carrier was ion-exchanged with 300 mL of the solution for 6 hours, filtered, washed with 200 mL of deionized water, dried, and calcined at 540° C. for 6 hours to obtain H-type MCM-56 zeolite. Mix 10g of H-type MCM-56 zeolite with 4.286g of γ-Al 2 o 3(Shanghai Petrochemical Research Institute) mixed, added 40mL of 4wt% nitric acid aqueous solution, kneaded, extruded, dried at 80°C for 12 hours, and roasted at 540°C in air for 6 hours to obtain a carrier. Analytical pure ammonium molybdate [(NH 4 ) 6 Mo 7 o 24 4H 2 O] 0.2210g and 10mL of water were made into an impregnating solution, impregnating the above-mentioned 3g carrier in equal volume at room temperature for 24 hours, drying at 120°C for 12 hours, and roasting at 500°C in air for 6 hours to obtain the catalyst, and the obtained weight ratio was: MoO 3 Catalyst C of / H type MCM-56 / alumina = 6 / 70 / 30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com