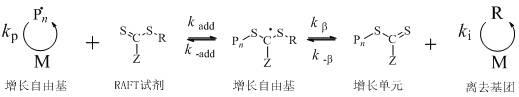
Preparation method for efficient chain transfer agent trithiocarbonate used for RAFT (reversible addition-fragmentation chain transfer) polymerization
A technology of chain transfer polymerization and addition-fragmentation, which is applied in organic chemistry and other fields, can solve the problems of complex preparation process, complicated post-processing, and difficulty in long-term storage, and achieve the effects of high product purity, convenient purification, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
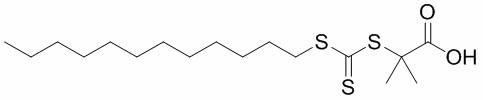
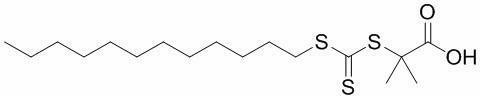
[0026] Add 2 g of potassium phosphate (K 3 PO 4 ) and 50mL of acetone, followed by 2g of carbon disulfide (CS 2 ) into a round-bottomed flask through a syringe, and stirred for 1 h; 2 g of dodecanethiol was added into a round-bottomed flask, and stirred for 1 h; finally, 1.5 g of 2-bromoisobutyric acid was added, and the reaction was carried out for 13 h. After the reaction, the solvent acetone was removed by distillation under reduced pressure, and the bright yellow trithioester C was obtained through dissolution, extraction and washing. 17 h 32 o 2 S 3 .
[0027] figure 1 Molecular formula for trithioester.
Embodiment 2
[0029] Add 15g potassium phosphate (K 3 PO 4 ) and 200mL of acetone, followed by 10g of carbon disulfide (CS 2 ) into a round-bottomed flask through a syringe, and stirred for 3 hours; 10 g of dodecanethiol was added into a round-bottomed flask, and stirred for 3 hours; finally, 7.5 g of 2-bromoisobutyric acid was added, and the reaction was carried out for 24 hours. The solvent after the reaction was removed by distillation under reduced pressure, and the bright yellow trithioester C was obtained by dissolving, extracting and washing. 17 h 32 o 2 S 3 .
Embodiment 3
[0031] Add 20g potassium phosphate (K 3 PO 4 ) and 500mL of acetone, followed by 20g of carbon disulfide (CS 2 ) into a round-bottomed flask through a syringe, and stirred for 4 hours; 20g of dodecanethiol was added into a round-bottomed flask, and stirred for 3 hours; finally, 15g of 2-bromoisobutyric acid was added, and the reaction was carried out for 24 hours. The solvent after the reaction was removed by distillation under reduced pressure, and the bright yellow trithioester C was obtained by dissolving, extracting and washing. 17 h 32 o 2 S 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com