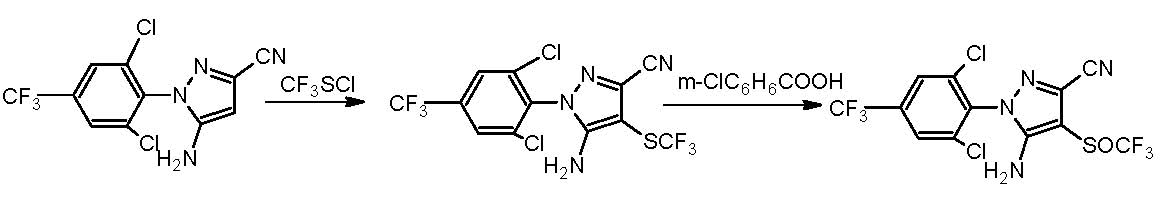
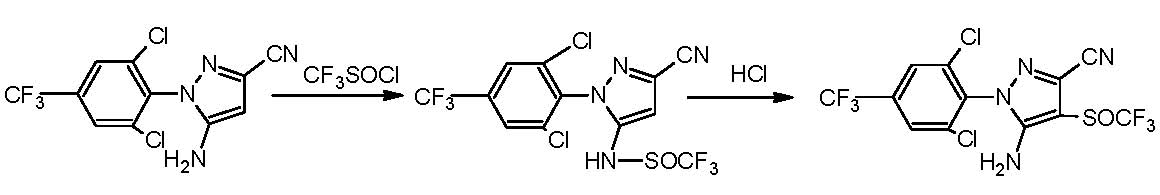
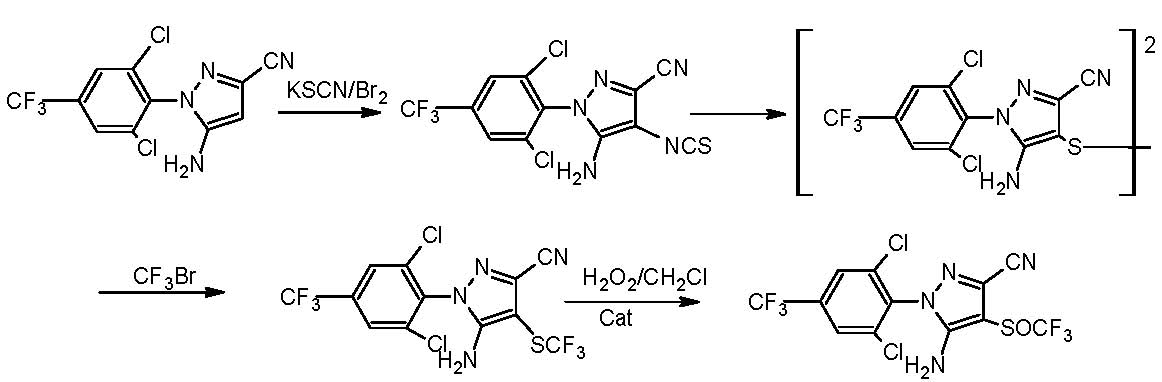
Synthesizing method for fipronil intermediates
A synthesis method and a technology for intermediates, which are applied in the field of chemistry, can solve the problems of low production yield of fipronil intermediates, difficult to industrialized production, complicated operations, etc., and achieve the effects of low production cost, simple method and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 0.3mol (ie 98.3g) of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole to 300ml of acetone, heat to 0.4 atm. 30 ~ 35 ℃, evaporate 50mL of acetone, then cool to 20 ℃, quickly add 0.24 moles of sulfur monochloride (ie 33.3g), and then react at 35 ℃ and under the negative pressure of -0.03--0.01MPa for 0.5 hours to obtain The mixture was allowed to stand at 35°C and 1 atmosphere for 0.5h, hydrogen chloride was removed, triethylamine was added dropwise to adjust pH=7, cooled to 5°C, filtered to obtain filtrate, the filtrate was washed with water, and dried to obtain 5-amino -1-(2,6-Dichloro-4-trifluoromethylphenyl)-3-cyanopyrazol-4-ylbissulfide 104.3g, content 97.0%, yield 95.8%, 1H NMR (300MHz, DMSO-d6): δ 7.98 (s, 4H, Ph), 6.69 (s, 4H, NH 2 ).
Embodiment 2
[0018] Add 0.3mol (ie 98.3g) of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole to 300ml of ethanol, heat to 0.6 atmospheres 65 ~ 70 ℃, evaporate 50mL of ethanol, then cool to 20 ℃, quickly add 0.18 moles of sulfur monochloride (ie 25g), and then react at 40 ℃ and under the negative pressure of -0.06--0.04MPa for 0.5 hours to obtain a mixture , the mixture was allowed to stand at 40°C and 1 atmosphere for 0.5h, hydrogen chloride was removed, and the PH=7 was adjusted with monomethylamine, cooled to 5°C, and the filtrate was filtered, washed with water, and dried to obtain 5-amino- 1-(2,6-Dichloro-4-trifluoromethylphenyl)-3-cyanopyrazol-4-ylbissulfide 101.4 g, content 96.8%, yield 93.0%.
Embodiment 3
[0020] Add 0.3mol (ie 98.3g) of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole to 300ml of acetonitrile, heat to 0.3 atm. 65~70 ℃, evaporate 50mL of acetonitrile, then cool to 20 ℃, quickly add 0.15 moles of sulfur monochloride (ie 20.8g), and then react for 0.5 hours at 40 ℃, under the negative pressure of -0.08--0.07MPa to obtain The mixture was allowed to stand at 45°C and 1 atmosphere for 0.5h, the hydrogen chloride was removed, the pH was adjusted to 7 with ammonia, cooled to 5°C, the filtrate was filtered, the filtrate was washed with water, and dried to obtain 5-amino-1 -(2,6-Dichloro-4-trifluoromethylphenyl)-3-cyanopyrazol-4-ylbissulfide 103.3 g, content 97.2%, yield 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com