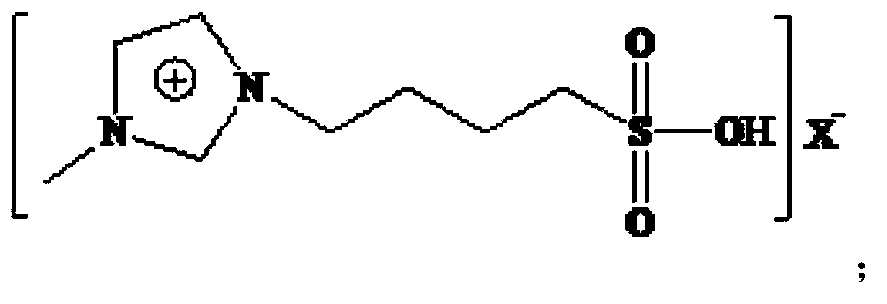
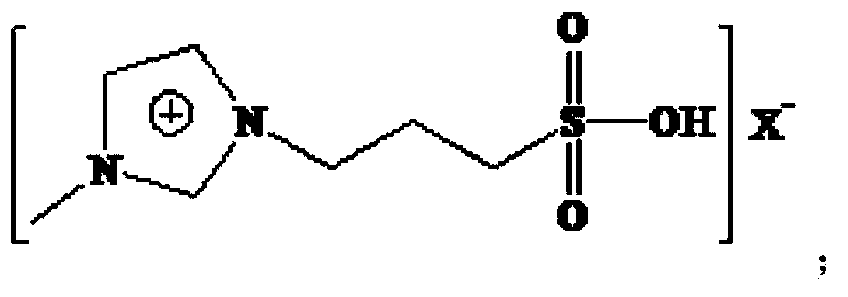
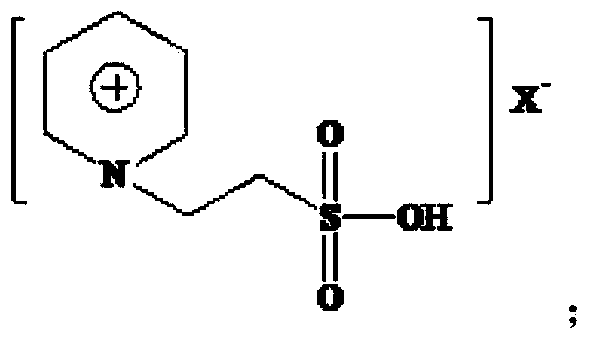
A process for preparing 2-ethyl-2-hexenal through the self-condensation of n-butyraldehyde catalyzed by an acidic ionic liquid
A technology of acidic ionic liquid and process method, applied in the field of green chemistry, can solve the problems of non-reusable liquid alkali catalyst, poor stability of solid alkali catalyst, high cost of three waste treatment, etc., achieve good industrial application prospects, and overcome the cumbersome post-treatment process , high thermal and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] other The preparation method of acidic ionic liquid is the same.
[0042] The Lewis acidic ionic liquid involved in the present invention is characterized in that it is a chloroaluminate-based acidic ionic liquid, prepared by the method described by Wikes et al. (Inorg.Chem., 1982, 21:1263). With [Bmim]Cl-AlCl 3 For example:
[0043] (1) Add the purified N-methylimidazole and chlorobutane into a three-necked flask at a molar ratio of 1:1-1:1.5, and react for 24-48 hours at a reaction temperature of 60-80°C , washed and dried to obtain light yellow viscous [Bmim]Cl.
[0044] (2) Under nitrogen protection, [Bmim]Cl was added to the three-necked upper bottle, and AlCl 3 The molar ratio of [Bmim]Cl to [Bmim]Cl is 1:1-3:1 and slowly add anhydrous AlCl in batches 3 , stir evenly, and react at 60-80°C until brown transparent [Bmim]Cl-AlCl is obtained 3 .
[0045] The preparation method of other Lewis acidic ionic liquids is the same.
Embodiment 1
[0047] In a 100ml autoclave, put 40g of n-butyraldehyde, and then add [Bmim]Cl-ZnCl with 1% n-butyraldehyde weight 2 ionic liquid, with N 2 Air replacement, magnetic stirring, and reaction at 150°C for 5 hours. After the reaction, the reaction solution was left to stand. After the reaction solution was separated, the upper layer liquid was taken for gas chromatography analysis. The conversion rate of n-butyraldehyde was 72.7%, and 2-ethyl The selectivity of -2-hexenal is 59.5%.
[0048] Examples 2-80 were carried out according to the operation steps of Example 1, and the reaction conditions and results are shown in the summary table.
[0049]
[0050]
[0051]
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com