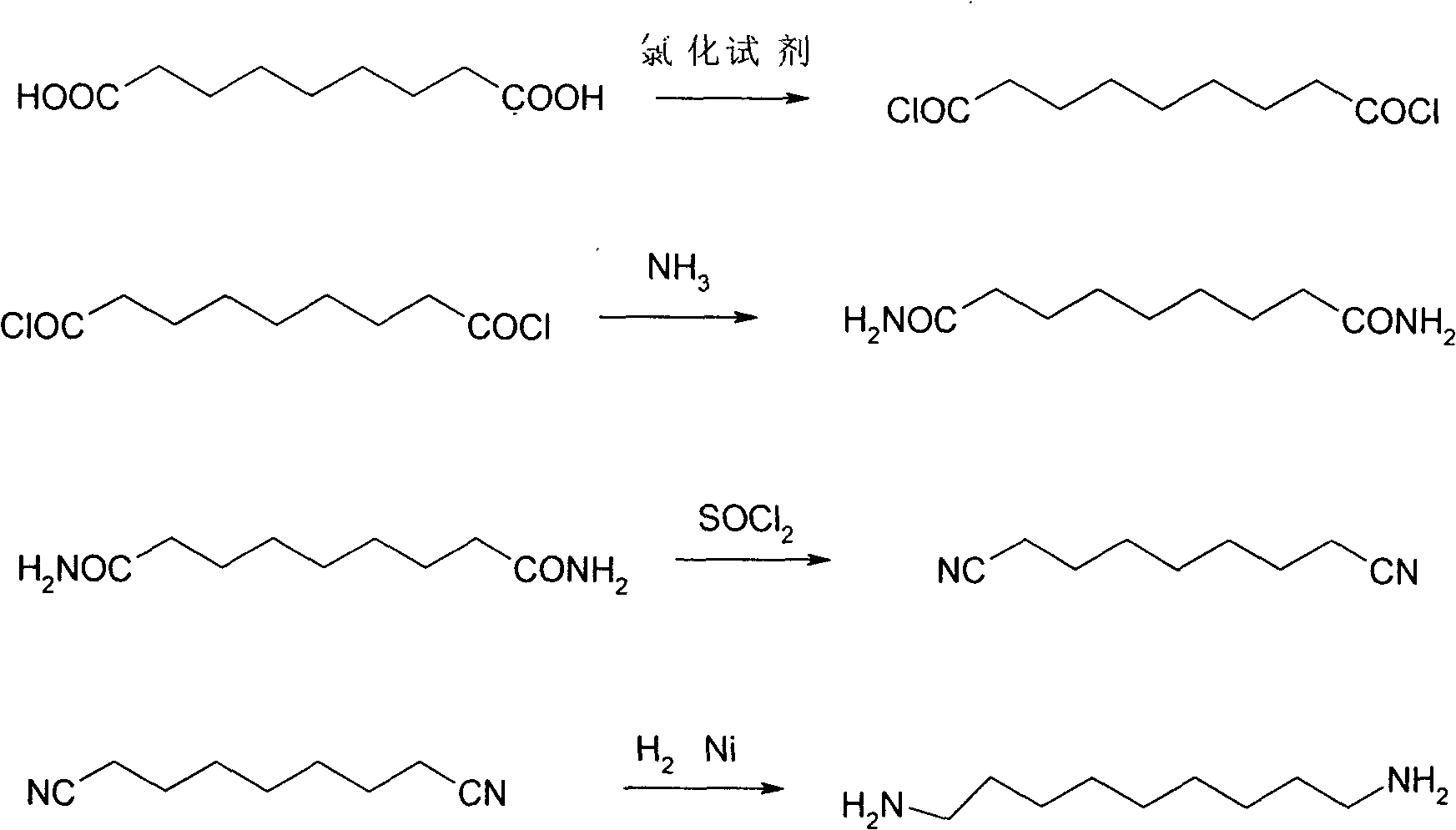
Method for preparing nonane diamine
A nonanediamine and azelaic amide technology, applied in the field of fine chemical intermediate preparation, can solve the problems of harsh production conditions, complicated equipment, etc., and achieve the effects of simple process, expanded application field, and convenient industrial operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Utilize N, N-dimethylformamide catalyst, sulfur oxychloride is solvent and chlorinating agent, prepares azelayl chloride
[0021] Using azelaic acid as raw material, add 18.8g of azelaic acid (0.1mol), 84.2g of phosphorus oxychloride (0.55mol) and 0.6g of N,N-dimethylformamide into a 250ml three-necked flask. Slowly raise the temperature of the mixture to 50° C., keep it warm for 1 hour, and then raise the temperature to reflux for about 5 to 6 hours. After the reaction was completed, excess thionyl chloride was distilled off. Add fresh toluene to obtain a toluene solution of azelayl chloride.
[0022] (2) Preparation of azelaic amide
[0023] In a 500ml beaker, add 50g of ice and 180g of 20% ammonia (relative to 10 times the mass of azelayl chloride) frozen in advance, at a temperature of -17°C, stir the azelayl chloride prepared in the example step (1) The toluene solution was added dropwise to the above-mentioned ammonia solution for about 0.5 hours. After the...
Embodiment 2
[0031] (1) Utilize N,N-dimethylformamide as a catalyst to prepare azelayl chloride in ethylene dichloride
[0032] Add 100 ml of dichloroethane, 0.6 g of N,N-dimethylformamide and 18.8 g (0.1 mol) of azelaic acid into a 250 ml three-necked flask. The mixture was stirred at room temperature, and 65.5 g (0.55 mol) of thionyl chloride was added dropwise. After the addition of thionyl chloride was completed, the reaction was heated and stirred at about 50°C for 1 hour, and the temperature was raised to reflux, and the reaction was about 5 to 6 Hour.
[0033] After the reaction was completed, after distilling off excess thionyl chloride and ethylene dichloride, 50 ml of fresh ethylene dichloride was added to obtain a solution of azelayl chloride in ethylene dichloride.
[0034] (2) prepare azelaic amide with ammonia water
[0035] In a 500ml beaker, add 50g of ice and 180g of 20% ammonia water frozen in advance, add the azelayl chloride dichloroethane solution prepared in step (1...
Embodiment 3
[0044] (1) Preparation of azelayl chloride in toluene using N,N-dimethylformamide catalyst In a 2500ml three-necked flask, 1000ml of toluene, 6g of N,N-dimethylformamide and 188g (1mol) of azelaic acid were added. The mixture was stirred at room temperature, and 655 g (5.5 mol) of thionyl chloride was added dropwise. After the addition of thionyl chloride was completed, the reaction was heated and stirred at about 50°C for 1 hour, and the temperature was raised to reflux, and the reaction was about 5~ 6 hours.
[0045] After the reaction was completed, excess thionyl chloride and toluene were distilled off, and 200 ml of fresh toluene was added to obtain a toluene solution of azelayl chloride.
[0046] (2) prepare azelaic amide with ammonia water
[0047] In a 5000ml beaker, add ice and 1800g of 20% ammonia water that has been frozen in advance, add the azelayl chloride toluene solution prepared by 3-(1) dropwise into the above ammonia solution for about 2 hours at -20°C, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com