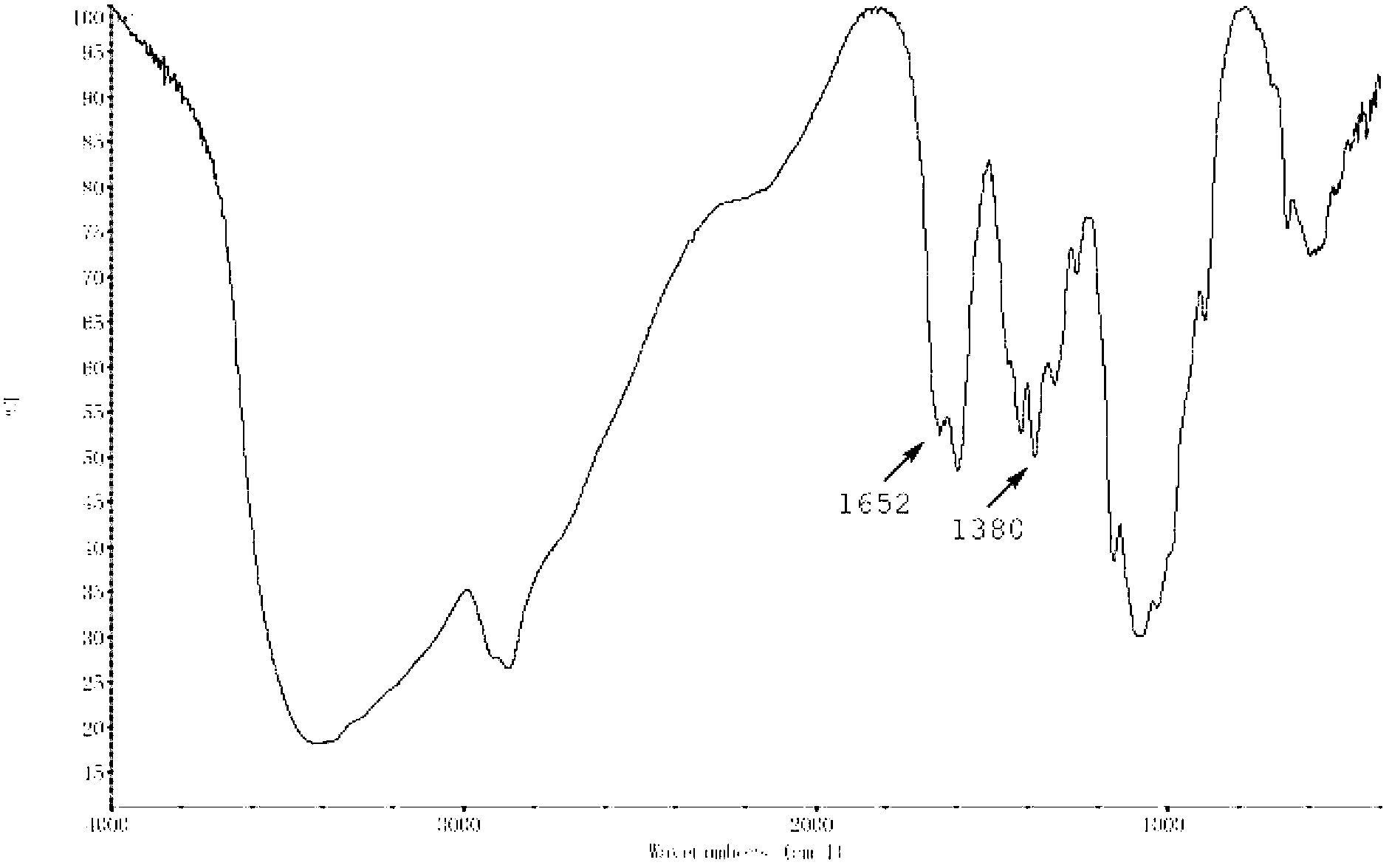
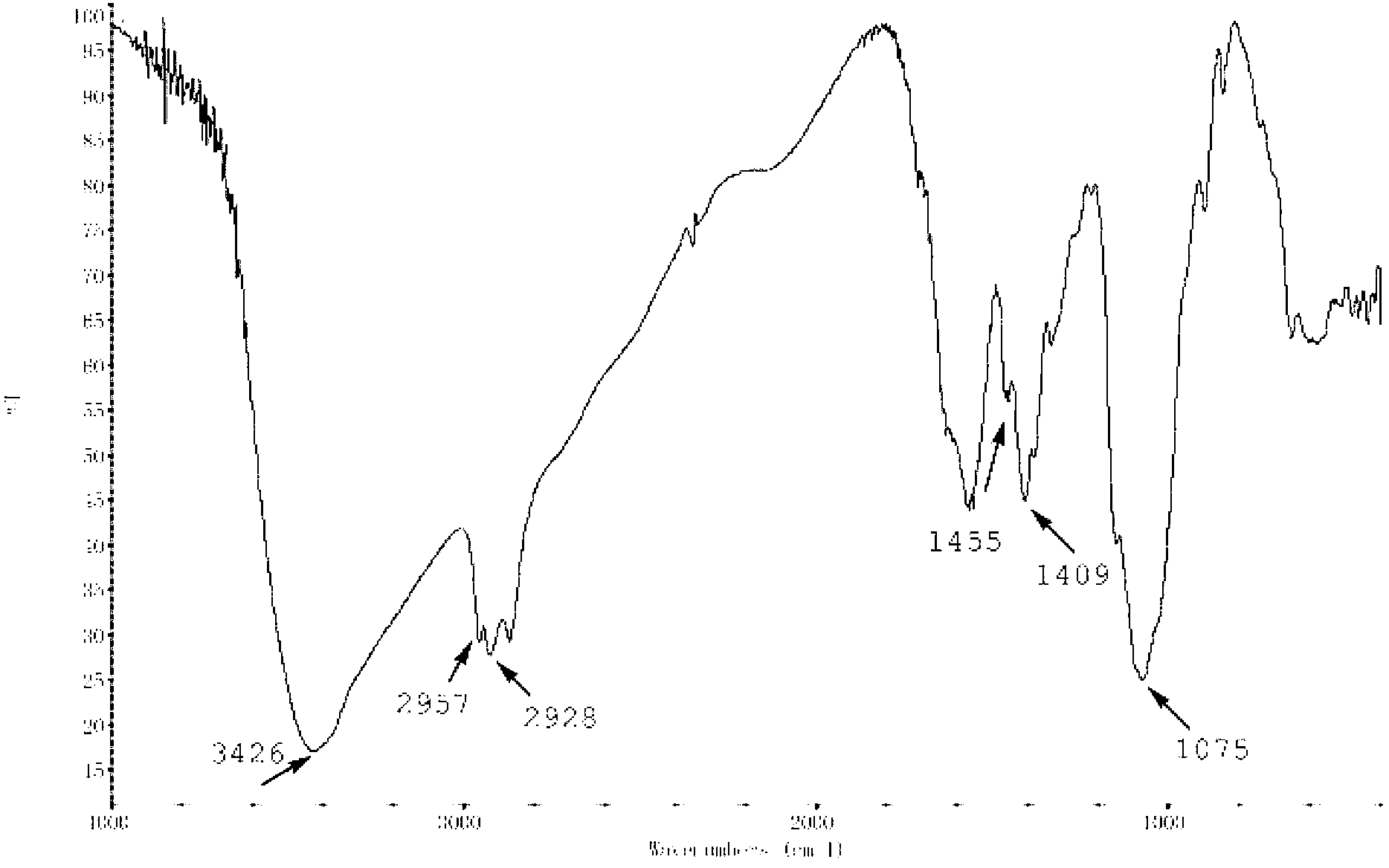
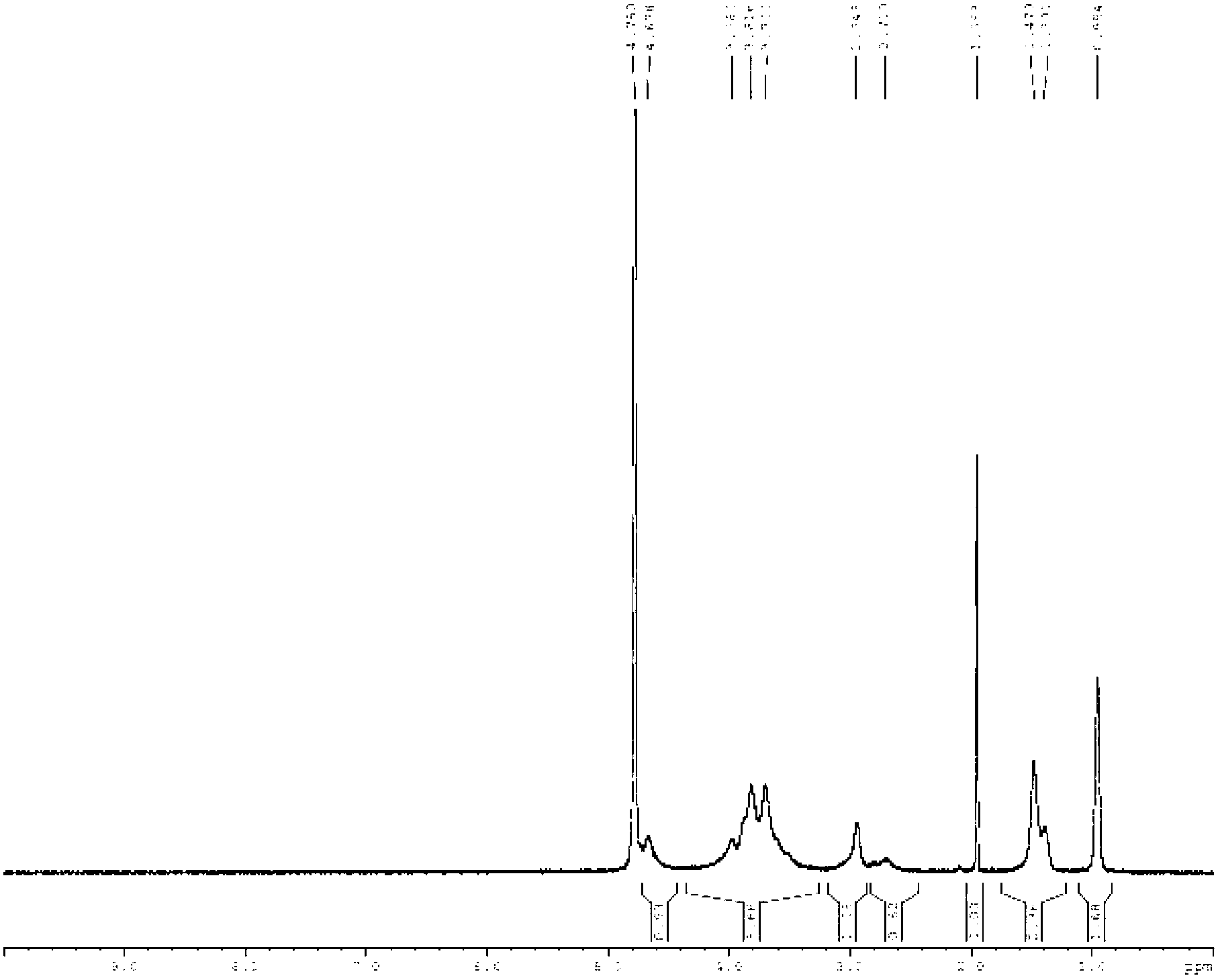
Thermo-sensitive chitosan derivative-hydroxypentyl chitosan and preparation method thereof
A technology of chitosan derivatives and hydroxyamyl shells, applied in the field of thermosensitive chitosan derivatives - hydroxyamyl chitosan and its preparation, to achieve the effect of simple preparation method and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of hydroxypentyl chitosan
[0029] The first step, the preparation of chitosan:
[0030] Take 100 grams of fresh shrimp shells, soak in 800 ml of 5% (W / V) hydrochloric acid to remove calcium, then soak in 800 ml of 10% (W / V) sodium hydroxide to remove impurities, and wash with deionized water PH to 7-8, drying and grinding to obtain powdered chitin, then add 800 ml of 50% (W / V) sodium hydroxide solution to deacetylation reaction at 90°C for 24 hours, wash the PH with deionized water value to 7, then dehydrated with 95% alcohol, and finally vacuum-dried to obtain chitosan with a deacetylation degree of 85%.
[0031] The second step, the purification of chitosan:
[0032] Add the chitosan prepared in the first step to 1000 ml of sodium hydroxide solution with a concentration of 2% (W / V), control the temperature at about 75°C, stir for 2 hours, filter with suction, and then add 800 ml of 2% ( W / V) sodium hydroxide solution, the same process...
Embodiment 2
[0038] Embodiment 2: the preparation of hydroxypentyl chitosan
[0039] The first step, the preparation of chitosan is with embodiment 1;
[0040] Second step, the purification of chitosan is with embodiment 1;
[0041] The 3rd step, the alkalization of chitosan is with embodiment 1;
[0042] The fourth step, the hydroxypentylation of chitosan:
[0043] Filter and extrude excess lye in 10 grams of alkalized chitosan with 100-mesh silk cloth, transfer to a three-necked bottle, add 80 ml of isopropanol and stir for 2 hours to completely disperse chitosan, then separate liquids Slowly add 200 ml of 1,2-epoxypentane dropwise into the funnel, stir in the container at a speed of 300 rpm at room temperature, react for 5 days, after the reaction is complete, precipitate with 9 times the volume of acetone, and wash with 75% ethanol pH value to 7-8, dehydration, and vacuum drying at 50°C to obtain white powdery hydroxypentyl chitosan.
Embodiment 3
[0044] Embodiment 3: the preparation of hydroxypentyl chitosan
[0045] The first step, the preparation of chitosan is with embodiment 1;
[0046] Second step, the purification of chitosan is with embodiment 1;
[0047] The third step, alkalization of chitosan:
[0048] Add 10 grams of purified chitosan into 100 milliliters of 50% (W / V) potassium hydroxide solution, and place it at room temperature for 24 hours.
[0049] The 4th step, the hydroxypentylation of chitosan is the same as example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com