Photosensitive resin and preparation method and application thereof
A technology of photosensitive resin and photoresist, which is applied in the direction of photomechanical equipment, photosensitive materials for photomechanical equipment, optics, etc., to achieve the effects of easy availability of raw materials, avoiding uneven lines, and good film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
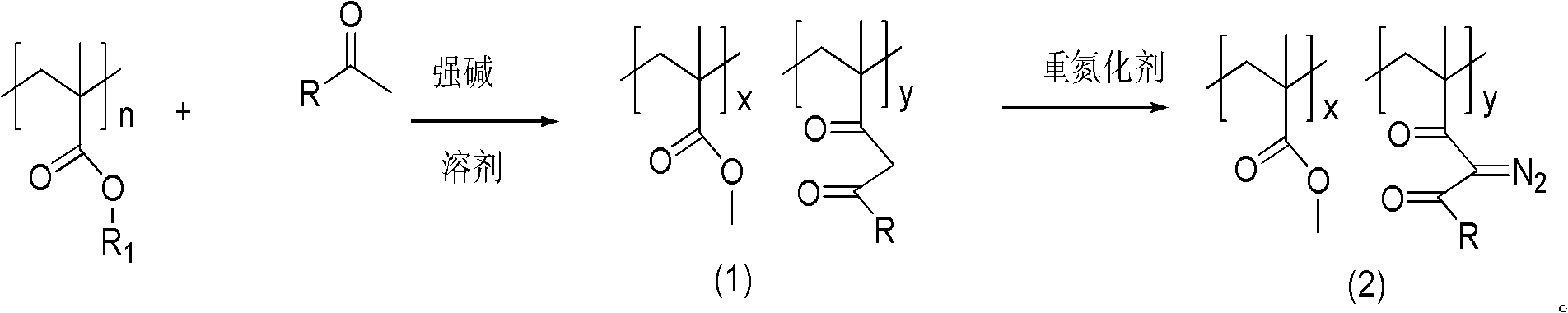
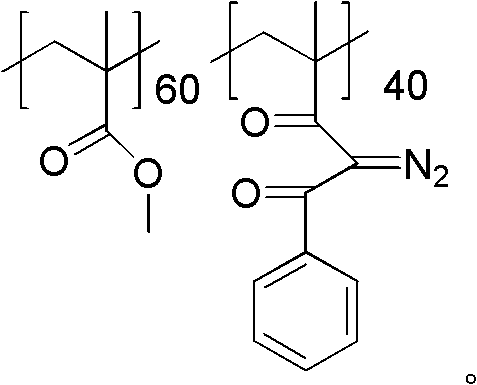
[0028] Dissolve 10 g of polymethyl methacrylate and 3.24 g (0.06 mol) of sodium methoxide in toluene, heat the system to weak reflux, and then slowly add 7.2 g (0.06 mol) of acetophenone dropwise to make the y value 40, drop Addition time was 2.5 hours. After dripping, continue to reflux for 2 hours, cool down, pour the reaction solution into methanol carefully, a solid precipitates out, filter the precipitate and dry it to obtain the aryl-substituted 1,3-diketone product. Then the aryl-substituted 1,3-diketone product was dissolved in acetonitrile, 7.88 g (0.04 mol) of p-toluenesulfonyl azide was added thereto, and after stirring at room temperature for 3.5 h, the reaction solution was poured into methanol , A solid precipitate was precipitated, and after filtering the precipitate, it was vacuum-dried at 30° C. for 24 hours to obtain a powdery photosensitive resin.
[0029] Infrared characterization results of powdered photosensitive resin: IR (KBr): 2143cm -1 (s, C=N=N), 1...
Embodiment 2
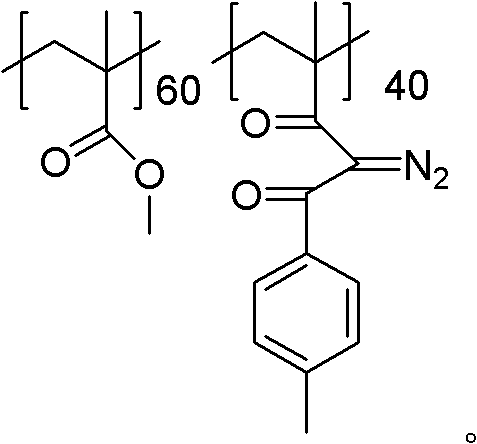
[0033] 10g of polymethyl methacrylate and 4.08g (0.06mol) of sodium ethylate were dissolved in methylene chloride, the system was heated to weak reflux, and then 8.04g (0.06mol) of p-methylacetophenone was slowly added dropwise, so that y The value was 40, and the dropping time was 3 hours. After dropping, continue to reflux for 4 hours, cool down, pour the reaction solution into ethanol carefully, a solid precipitates out, filter the precipitate and dry it to obtain the aryl-substituted 1,3-diketone product. Then the aryl-substituted 1,3-diketone product was dissolved in acetonitrile, 7.88 g (0.04 mol) of p-toluenesulfonyl azide was added thereto, and after stirring at room temperature for 5 h, the reaction solution was poured into ethanol, A solid precipitated out. After filtering the precipitate, it was vacuum-dried at 30° C. for 24 hours to obtain a powdery photosensitive resin.
[0034] Infrared characterization results of powdered photosensitive resin: IR (KBr): 2143cm ...
Embodiment 3
[0038] Dissolve 10 g of polymethyl methacrylate and 3.24 g (0.06 mol) of sodium methoxide in tetrahydrofuran, heat the system to weak reflux, and then slowly add an appropriate amount of 9.0 g (0.06 mol) of p-methoxyacetophenone, so that y The value is 40, so that the y value is 40, and the dropping time is 1.5 hours. After dripping, continue to reflux for 2 hours, cool down, pour the reaction solution into methanol carefully, a solid precipitates out, filter the precipitate and dry it to obtain the aryl-substituted 1,3-diketone product. Then the aryl-substituted 1,3-diketone product was dissolved in acetonitrile, 7.88 g (0.04 mol) of p-toluenesulfonyl azide was added thereto, and after stirring at room temperature for 3.5 h, the reaction solution was poured into methanol , a solid precipitated out, and the precipitate was filtered and vacuum-dried at 30° C. for 24 hours to obtain a powdery photosensitive resin.
[0039] Infrared characterization results of powdered photosens...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com