Preparation method of medical rubber stopper
A production method and technology of rubber stoppers, which are applied in the field of production of high-performance medical halogenated butyl rubber stoppers, can solve the problems of cumbersome manufacturing process, complex formula, and compatibility, and meet the requirements of special drug compatibility, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1: A kind of production method of medical rubber stopper, the rubber stopper is immersed in the alkaline aqueous solution for treatment; The rubber stopper treated in the alkaline aqueous solution is immersed in the acidic aqueous solution for neutralization treatment; The acidic aqueous solution is neutralized Rinse the rubber stopper with purified water or water for injection, and add silicon oil for siliconization treatment; dry the siliconized rubber stopper at 115~130°C to obtain a medical rubber stopper that can meet the compatibility requirements of special drugs.
[0012] The alkaline aqueous solution is sodium hydroxide or sodium carbonate with a concentration of 0.05-3% by mass; the temperature for immersion in the alkaline aqueous solution is 110-140° C., and the time is 30-90 minutes. The acidic aqueous solution is an aqueous solution of hydrochloric acid solution with a mass percentage concentration of 0.05 to 0.25% and a 0.1% mass percentage conc...
Embodiment 2
[0014] Example 2: A production method of medical rubber stoppers. A certain amount of halogenated butyl rubber stoppers is treated in sodium hydroxide (NaOH) with a concentration of 0.20% by mass at 121° C. for 60 minutes.
[0015] The above-mentioned rubber stopper treated with an alkaline aqueous solution is mixed with a hydrochloric acid solution of 0.20% by mass percentage concentration and an aqueous solution of disodium edetate with a concentration of 0.2% by mass percentage, hydrochloric acid solution and disodium edetate in the aqueous solution The volume ratio of the aqueous solution is 1:0.05, and it is treated at 50°C for 10 minutes.
[0016] The rubber stopper treated with the acidic aqueous solution was rinsed with water for injection and siliconized with 350 cst of simethicone.
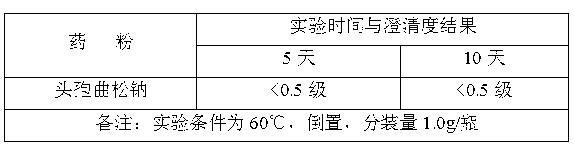
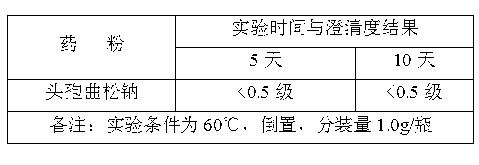
[0017] The siliconized rubber stopper was dried at 115° C. for 60 minutes. The compatibility test results of the obtained high-performance medical rubber stopper and ceftriaxone sodium ...
Embodiment 3
[0020] Embodiment 3: A production method of medical rubber stoppers, treating halogenated butyl rubber stoppers in sodium hydroxide (NaOH) with a concentration of 0.20% by mass at 121° C. for 60 minutes.
[0021] The above-mentioned rubber stopper treated with an alkaline aqueous solution is mixed with an aqueous solution of 3% phosphoric acid and a 0.1% aqueous solution of disodium edetate in a concentration of 3% by mass, phosphoric acid solution and disodium edetate in the aqueous solution The volume ratio of the aqueous solution is 1:0.05, and it is treated at 50°C for 10 minutes.
[0022] The rubber stopper treated with the acidic aqueous solution was rinsed with water for injection and siliconized with 500 cst of simethicone.
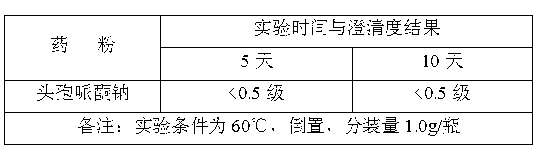
[0023] The siliconized rubber stopper was dried at 115° C. for 60 minutes. The compatibility test results of the obtained high-performance medical rubber stopper and ceftriaxone sodium for injection are shown in Table 2.
[0024] Table 2
[002...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com