Method for preparing diacetylacyclovir with 7-bit diacetylacyclovir
A technology of diacetyl acyclovir and butanediol diethyl ester, which is applied in the field of synthesis of pharmaceutical and chemical intermediates, can solve the problems of only 91% product purity, unfavorable industrial production, and low product purity, so as to save manpower and material resources, Improved resource utilization and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
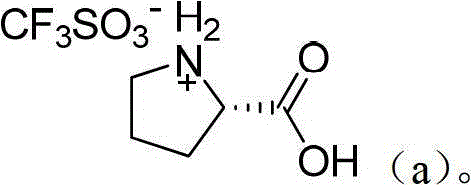
[0031] Preparation of catalyst organic ammonium trifluoromethanesulfonate: as shown in Table 1 below, add 20 mmol of organic amines into a 100 mL single-necked bottle equipped with a constant pressure dropping funnel and magnetic stirring, and dissolve with 50 mL of toluene. Under ice bath, 3.0 g (20 mmol) of trifluoromethanesulfonic acid was added dropwise. After the dropwise addition, the mixture was stirred and reacted under ice bath for 0.5 h, and a light blue solid was precipitated. After suction filtration, the filter cake was vacuum-dried at 60°C to obtain the corresponding organic ammonium trifluoromethanesulfonate catalyst.
[0032] Table 1
[0033] catalyst
[0034] Diphenylamine triflate
Embodiment 1
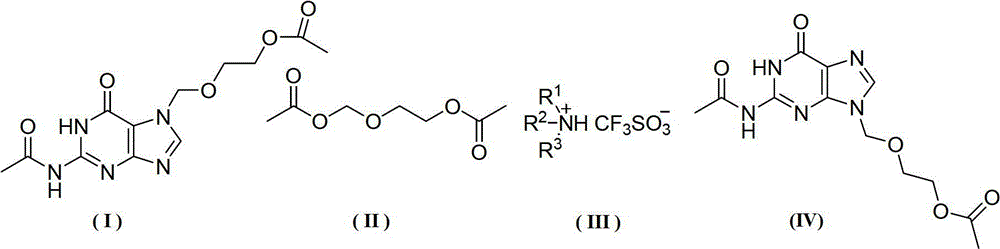
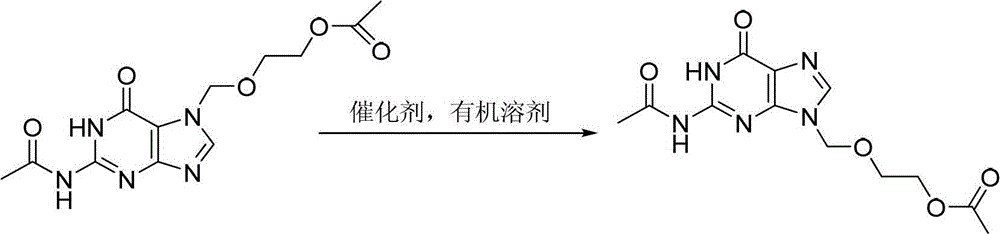
[0036] Add 15.4g (0.05mol) 7-DACV, 0.96g (3.0mmol) diphenylamine triflate, 1.76g (0.01mol) 2-oxa-1 into a 500mL three-necked flask equipped with mechanical stirring and a thermometer , 4-butanediol diethyl ester, 10.2g (0.10mol) acetic anhydride, , 100mL chloroform, start heating up, react at 70°C for 25h, after the reaction is over, cool, filter, wash the filter cake with water until the filtrate becomes clear, Then the filter cake was dried to obtain 12.4 g of diacetyl acyclovir, the yield was 80.0%, and the HPLC detection content was 97.6%.
Embodiment 2
[0038]Add 15.4g (0.05mol) 7-DACV, 1.82g (6.0mmol) diphenylamine trifluoromethanesulfonate, 17.6g (0.1mol) 2-oxa-1 into a 500mL three-necked flask equipped with mechanical stirring and a thermometer , 4-butanediol diethyl ester, 20.4g (0.20mol) acetic anhydride, 150mL xylene, start heating up, react at 90°C for 20h, after the reaction is over, cool, filter, wash the filter cake with water until the filtrate becomes clear, Then the filter cake was dried to obtain 13.0 g of diacetyl acyclovir, the yield was 84.8%, and the HPLC detection content was 95.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com