Novel 1,2,4-triazole derivative of chitosan and preparation method thereof
A chitosan and triazole technology, applied in the field of marine chemical engineering, can solve problems such as limited application and gap, and achieve the effects of improving biological activity, overcoming poor solubility and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 chitosan methyl 1,2, the preparation of 4-triazole derivative (1)
[0028] Add 1.30 grams of acetylthiourea chitosan with a molecular weight of 230,000 into a three-necked flask, add 20 mL of absolute ethanol, dropwise add 0.3 mL of acetic anhydride, heat to 60 ° C, and dropwise add hydrazine hydrate (1.0 mL) to it. 10 mL of ethanol solution, after the dropwise addition, the temperature was raised to reflux, and the reaction was carried out for 10 hours. The reactant was cooled to room temperature, filtered with suction, washed with absolute ethanol, and dried at 60° C. to obtain 0.70 g of white powder, namely chitosan methyl 1,2,4-triazole derivative.
[0029] The preparation of the acylthiourea chitosan can be found in the following documents: Zhimei Zhong, Ronge Xing, Song Liu, Lin Wang, Shengbao Cai, Pengcheng Li.Synthesis of acylthiourea derivatives of chitosan and their antimicrobial activities in vitro[J].Carbohydr Res, 2008, 343: 567-570.
[0030]...
Embodiment 2
[0031] Embodiment 2 chitosan chloromethyl 1,2, the preparation of 4-triazole derivative (2)
[0032] Add 4.41 grams of chloroacetylthiourea chitosan with a molecular weight of 230,000 into a three-necked flask, add 60 mL of absolute ethanol, dropwise add 0.9 mL of acetic anhydride, heat to 60 ° C, and dropwise add hydrazine hydrate (3.0 mL) 30 mL of ethanol solution, the temperature was raised to reflux after the dropwise addition, and the reaction was carried out for 10 hours. The reactant was cooled to room temperature, filtered with suction, washed with absolute ethanol, and dried at 60°C to obtain 3.30 g of yellow powder, namely chitosan chloromethyl 1,2,4-triazole derivative.
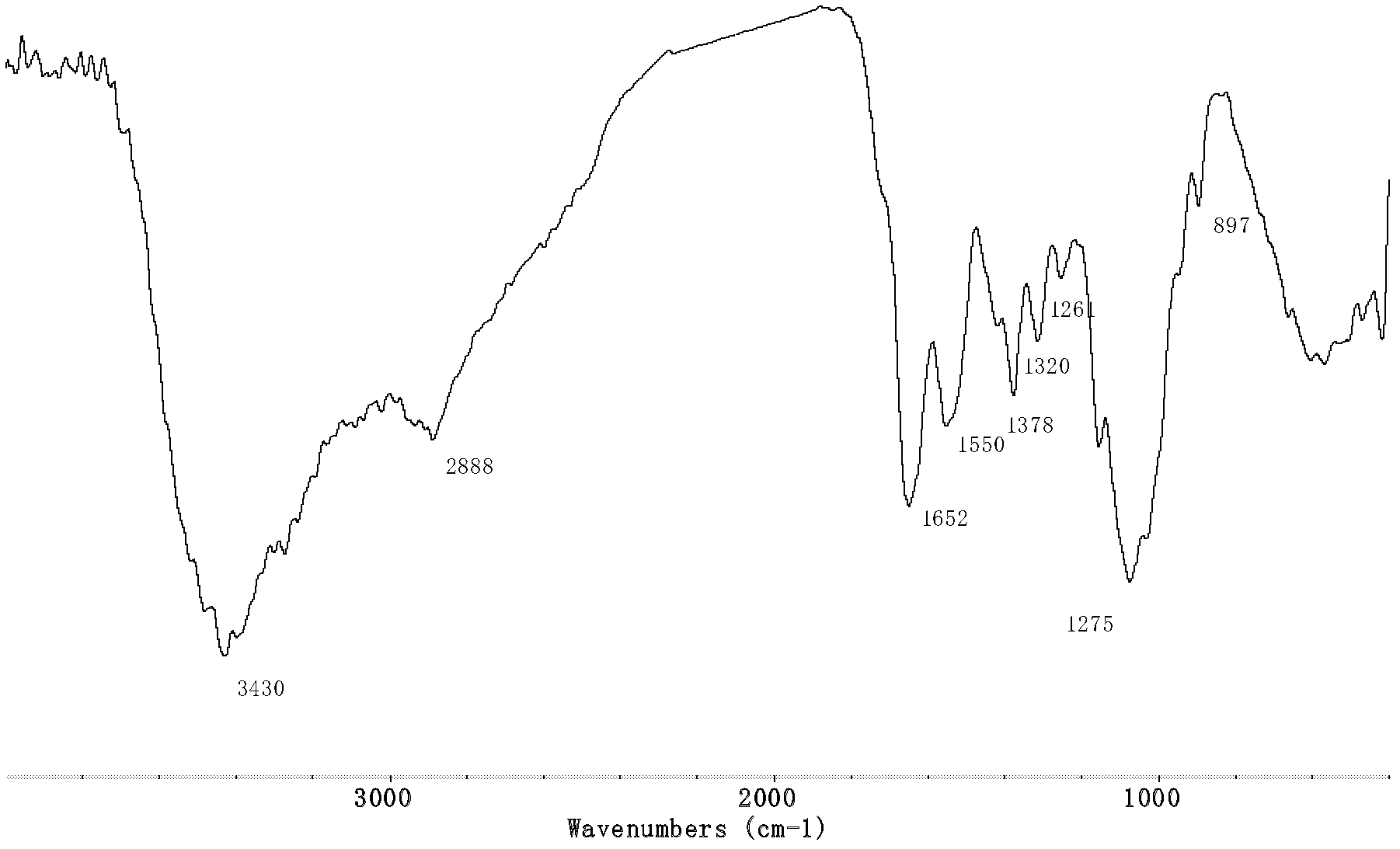
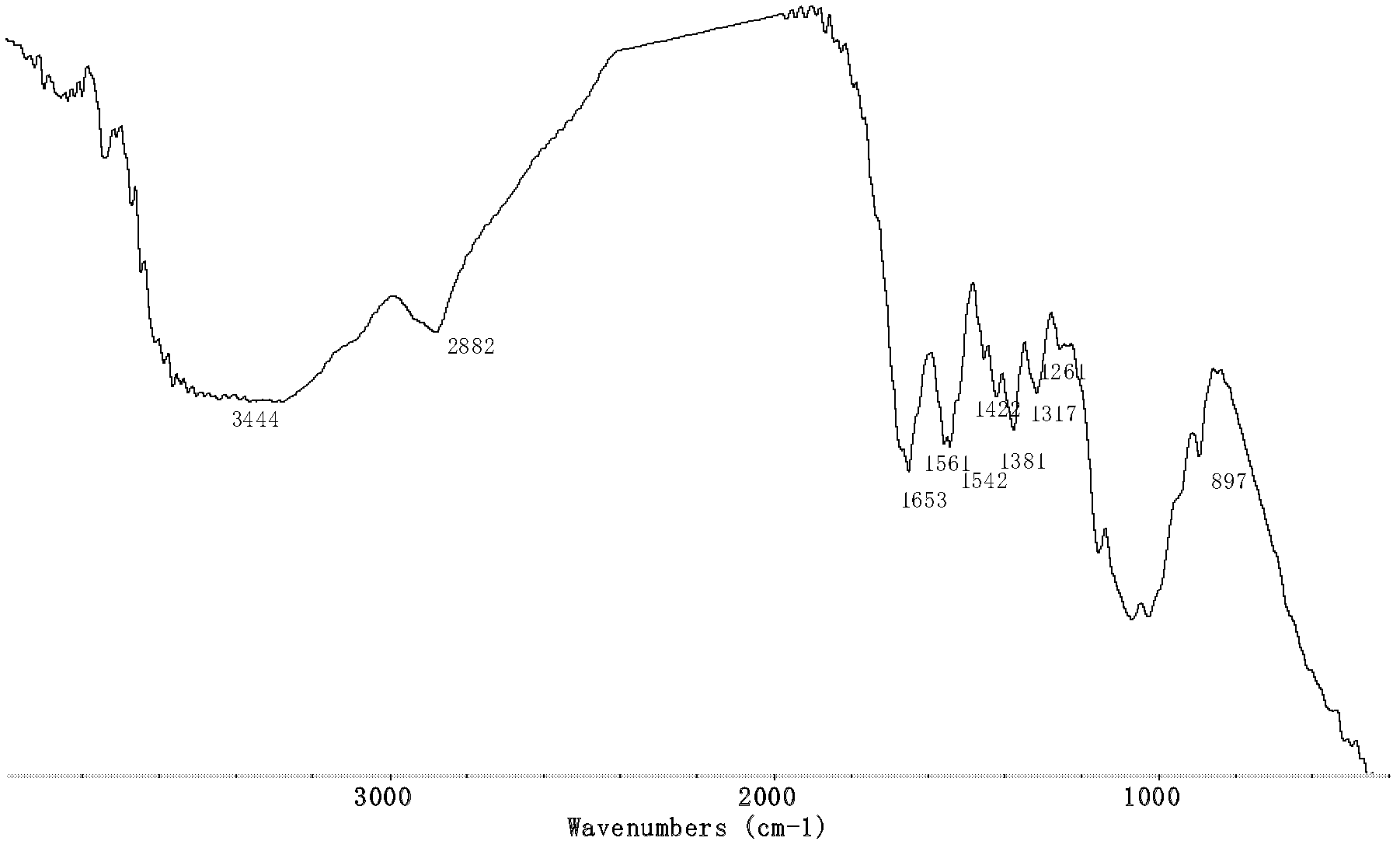
[0033] Infrared spectrum shows chitosan chloromethyl 1,2, the infrared spectrogram of 4-triazole derivative ( Figure 4 ) and the infrared spectrum of chloroacetylthiourea chitosan ( image 3 ) compared to that at 1258cm -1 The C=S absorption peak that appears becomes weaker obviously, and at th...
Embodiment 3
[0034] Embodiment 3 chitosan phenyl 1,2, the preparation of 4-triazole derivative (3)
[0035] Add 1.60 grams of benzoylthiourea chitosan with a molecular weight of 230,000 to a three-necked flask, add 20 mL of absolute ethanol, drop 0.3 mL of acetic anhydride, heat to 60 ° C, dropwise add hydrazine hydrate (1.0 mL ) of ethanol solution 10mL, the dropwise addition was completed and the temperature was raised to reflux, and the reaction was carried out for 10 hours. The reactant was cooled to room temperature, filtered with suction, washed with absolute ethanol, and dried at 60°C to obtain 1.0 g of white powder, namely chitosan phenyl 1,2,4-triazole derivative.
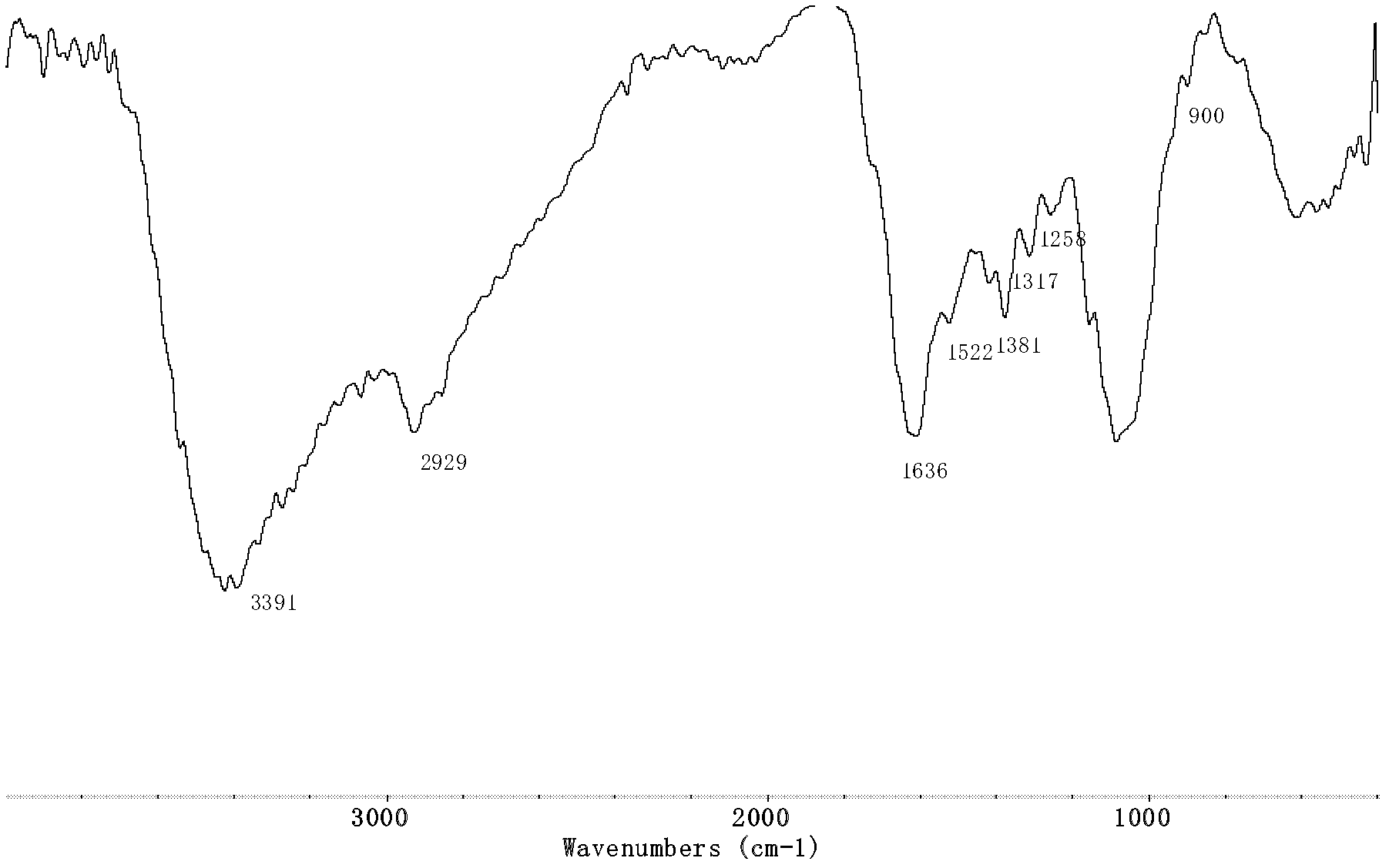
[0036] Infrared spectrum shows: the infrared spectrogram of chitosan phenyl 1,2,4-triazole derivative ( Figure 6 ) and the infrared spectrum of benzoylthiourea chitosan ( Figure 5 ) compared to that at 1261cm -1 The C=S absorption peak that appears becomes weaker obviously, and at the same time the 1656cm -1 The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com