A method for producing ethylene glycol by catalytic conversion of cellulose inhibiting the generation of cyclic ether alcohols
A technology of catalytic conversion and cellulose, applied in chemical instruments and methods, organic chemistry, preparation of hydroxyl compounds, etc., can solve the problems of difficult rectification separation and removal, reduce the difficulty of rectification separation, reduce the generation of by-products, and achieve high yields. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 10kg of corn stalk powder (20-40 mesh), add water to make the water content 30wt%, put it in a steam explosion reactor at 160°C, (pressure 1.0MPa) constant pressure for 60 seconds, and then perform steam explosion operation. To the obtained 8kg solid residue (dry weight), add 50kg concentration of 1wt% NaOH aqueous solution to it, soak 12h under room temperature 25 ℃, add 50kg concentration wherein to it after filtering out and be 1wt% hydrogen peroxide, soak 12h at room temperature, then Rinse with clear water until neutral to obtain 5 kg (dry weight) of corn stalk cellulose raw material.
[0020] The corn stalk powder is replaced by sorghum stalk, and the corresponding cellulose raw material can be obtained according to the same method as above.
Embodiment 2
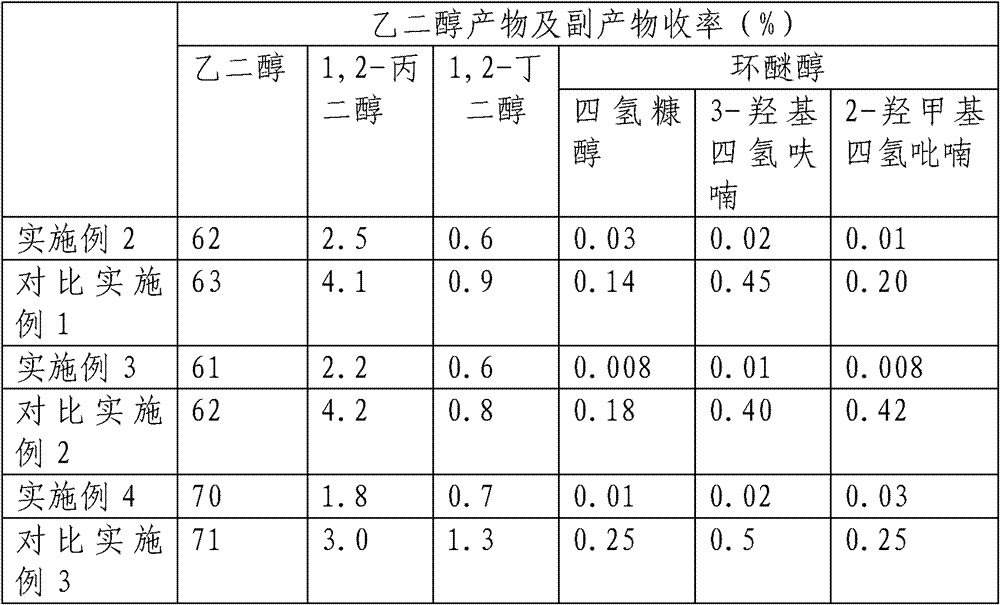
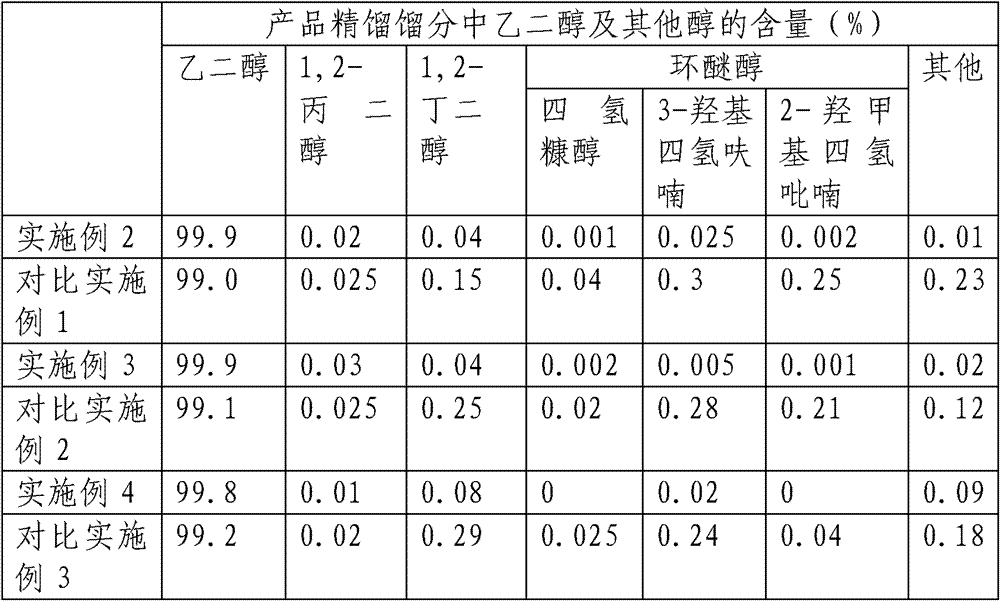
[0022] Get 10.0g corn stalk cellulose (gained in embodiment 1) and add 100ml water, 0.1g tungstic acid, 0.1g 5%Ru / AC catalyst, 0.05g 0.5%Ir-1%ReOx / AC (0<x≤3.5) The reaction was carried out in a high-pressure reactor at 250° C. for 2 hours, the stirring speed was 500 rpm, and the hydrogen pressure was 7 MPa during the reaction. After the reaction is finished, cool down to room temperature, release the pressure, open the kettle and centrifuge to obtain a liquid product, and analyze the yield of polyol product and cyclic alcohol by-product by gas chromatography.
Embodiment 3
[0026] Get 10.0g sorghum stalk cellulose (gained in embodiment 1) and add 100ml water, 0.1g ammonium metatungstate, 0.1g Raney nickel catalyst, 0.05g 2%Ru-0.2%Re / SiO 2 The reaction was carried out in a high-pressure reactor at 240° C. for 2 hours, the stirring speed was 500 rpm, and the hydrogen pressure was 7 MPa during the reaction. After the reaction is finished, cool down to room temperature, release the pressure, open the kettle and centrifuge to obtain a liquid product, and analyze the yield of polyol product and cyclic alcohol by-product by gas chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com