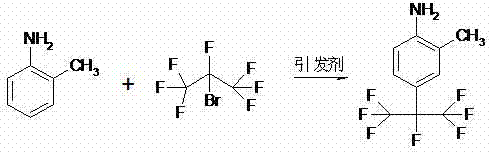
Method for preparing 2-methyl-4-(1,1,1,2,3,3,3-heptafluoro-2-propyl) aniline
A technology of methyl aniline and methyl, which is applied in the field of preparation of perfluoroalkylaniline derivatives, and can solve problems such as safety and insecurity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
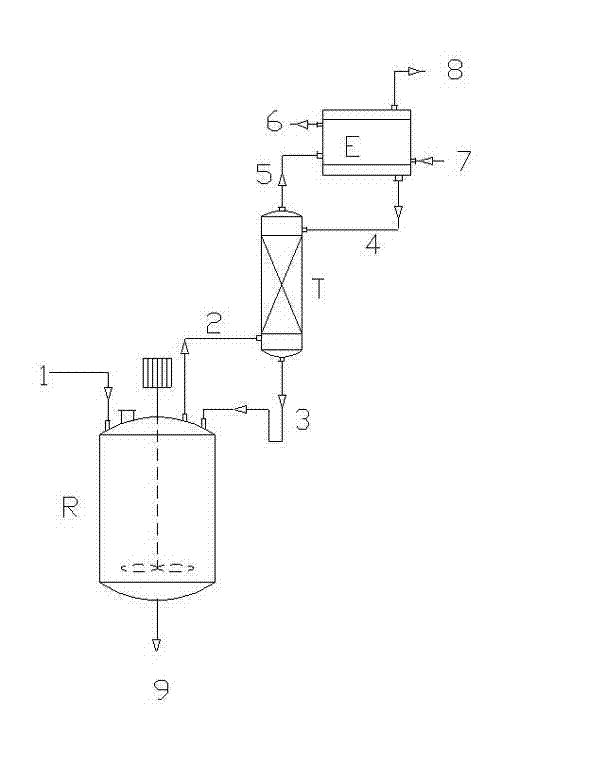
[0052]Add the liquid mixture of 20ml water and 20ml tert-butyl methyl ether in 1 liter closed reactor, then add 1 gram (9.3mmol) 2-methylaniline, 2.3 gram (12.5 mmol) sodium dithionite, 1.1 gram (13 mmol) Sodium bicarbonate and 0.4 gram (1.2 mmol) tetrabutylammonium bisulfate, start stirring, open the frozen brine of condenser, make the internal temperature of condenser be controlled at-20 ℃, control reactor internal temperature at 20-30 ℃, Continuously and slowly add 3.1 grams (12.5 mmol) of 1,1,1,2,3,3,3-heptafluoro-2-bromopropane with a micro-feed pump, vent the tail gas of the condenser into the tail gas treatment system, and the resulting mixture Stir at room temperature for 2 hours. Separate the organic layer, extract the aqueous layer with 20ml ethyl acetate, combine the extract with the organic layer, and wash with 2N aqueous hydrochloric acid, 5% sodium carbonate and saturated sodium chloride solution successively, and wash the organic layer with anhydrous magnesium s...
Embodiment 2
[0055] Open the manhole cover of 1000 liters of glass reaction, add the mixture of 200 liters of water and 200 liters of methyl tert-butyl ether in the reactor, then add 27.5 kg of 2-methylaniline, 40 kg of sodium dithionite, 24 kg of sodium bicarbonate and 4 kg of tetrabutylammonium bisulfate, turn on the motor to stir after adding, and after mixing evenly, turn on the frozen brine of the condenser to control the internal temperature of the condenser at -20°C, and the exhaust gas of the condenser Vent to the tail gas treatment system, control the internal temperature of the reactor between 20-30 ℃, start to continuously inject 67 kg of 1,1,1,2,3,3,3-heptafluoro-2 with a metering pump -bromopropane, the system pressure in the reactor is below 0.5 bar during the reaction, and the rear system pressure is normal pressure, and the time for pumping 1,1,1,2,3,3,3-heptafluoro-2-bromopropane is 0.5-4 hours, after feeding, keep warm for 4 hours. The reactants were separated into layer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com