Substituted quinoline-2-formaldehyde-phenylhydrazone derivative, and preparation method and application thereof
A compound, quinoline technology, applied in the fields of medicinal chemistry and pharmacotherapeutics, which can solve the problems of insignificant experimental effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
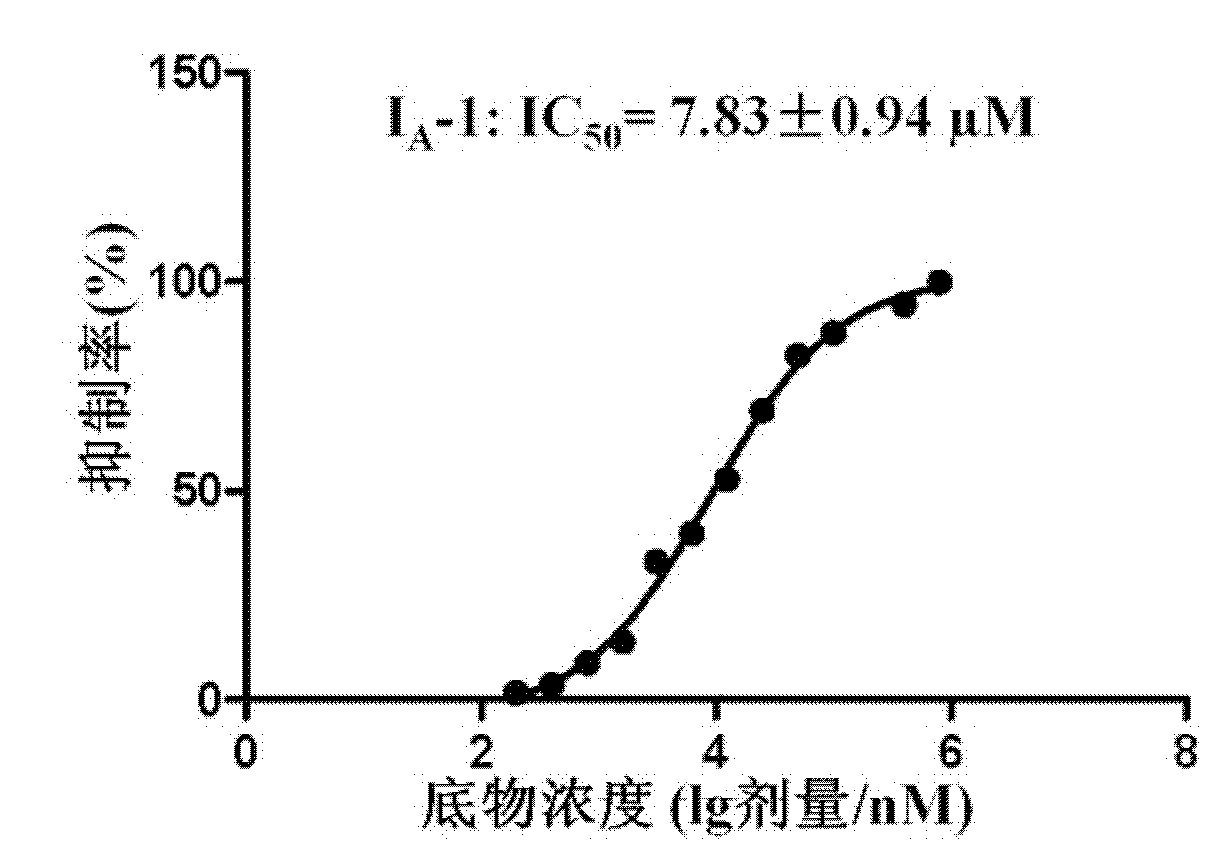
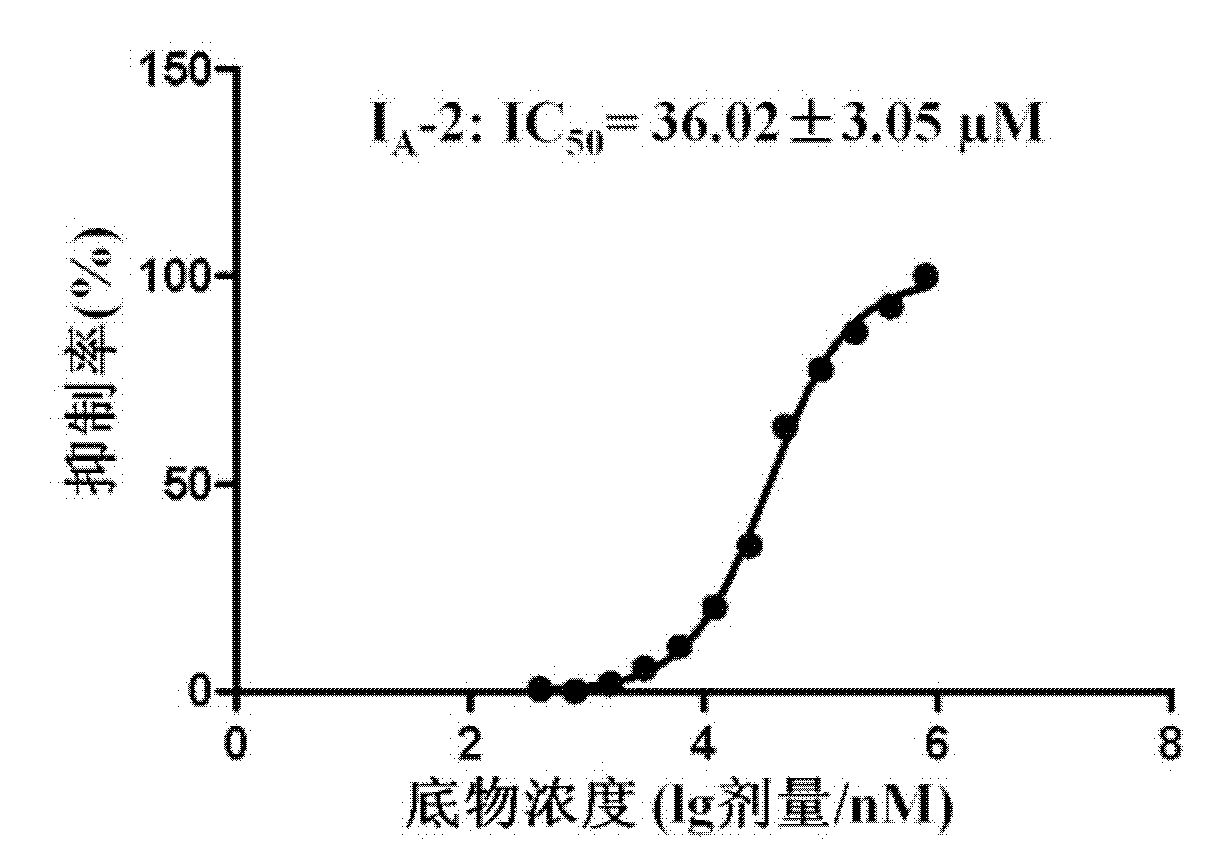
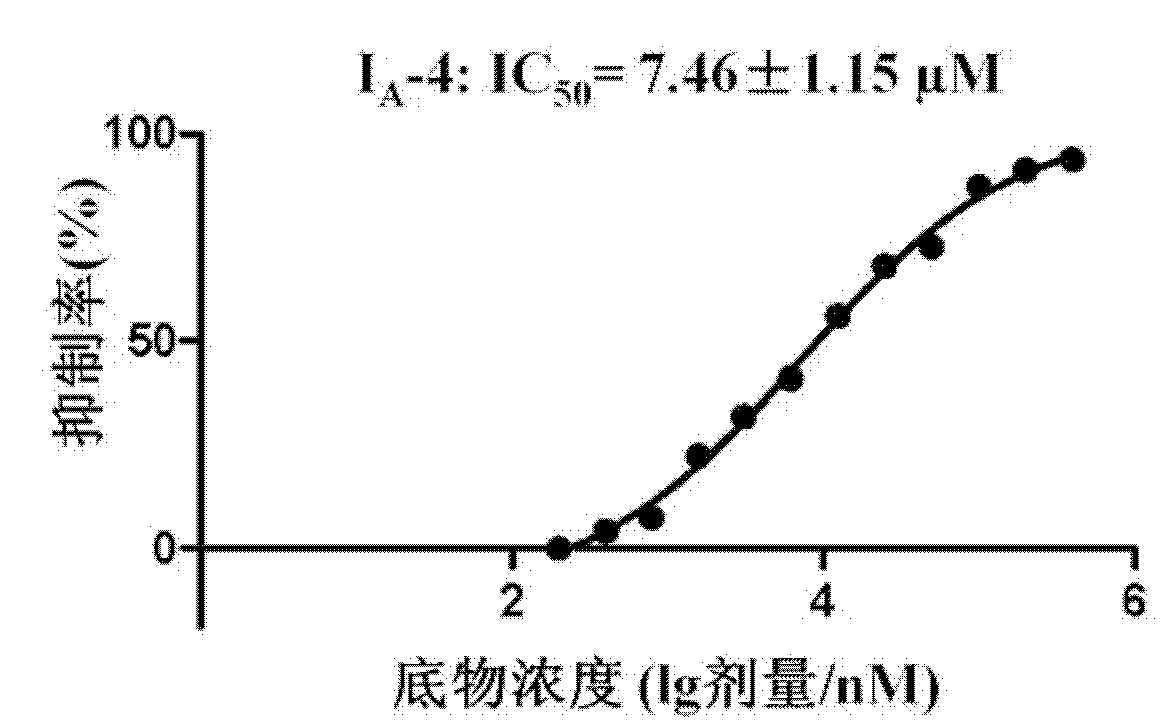
Image
Examples
Embodiment 1
[0072] Example 1 Preparation of 2-methyl-quinoline-4-carboxylic acid (intermediate II)
[0073]
[0074] Under the protection of nitrogen, 1.0 grams of isatin was put into potassium hydroxide solution (potassium hydroxide: 2.67 grams, water: 5 milliliters), reflux reaction at 50 ° C for 15 hours, 5 ml of acetone was added dropwise, and the reaction was completed for 3 hours. The reaction solution was cooled to room temperature, adjusted to pH=7 with concentrated hydrochloric acid, and a small amount of potassium chloride solid was formed, which was removed by suction filtration. Then the pH of the mother liquor was adjusted to 3, and a large amount of milky white solid was precipitated, which was suction filtered, washed with water, and dried to obtain intermediate II as a white solid with a yield of 50%. Mp 248-250°C; 1 H NMR (DMSO, 400MHz): δ2.72(s, 3H), 7.64(t, 1H), 7.78(t, 1H), 7.84(s, 1H), 8.02(d, 1H), 8.63(d, 1H ).
Embodiment 2
[0075] Example 2 Preparation of ethyl 2-methyl-quinoline-4-carboxylate (intermediate III)
[0076]
[0077] Put 1.0 g of 2-methyl-4-quinolinecarboxylic acid (intermediate II) into 30 ml of ethanol, add 2 ml of concentrated sulfuric acid dropwise, mix and stir at 80° C. for 18 hours, and evaporate the solvent to obtain 0.86 g of 2-methanol Ethyl-4-quinolinecarboxylate (Intermediate III) is a white solid with a yield of 80%. 1 H NMR (CDCl 3 , 400MHz) δ1.50(t, 3H), 2.83(s, 3H), 4.53(q, 2H), 7.61(t, 1H), 7.76(t, 1H), 7.82(s, 1H), 8.10(d , 1H), 8.71(d, 1H).
Embodiment 3
[0078] Example 3 Preparation of ethyl 2-formyl-quinoline-4-carboxylate (intermediate IV)
[0079]
[0080] Put 0.50 g of ethyl 2-methyl-4-quinolinecarboxylate (intermediate III) into 10 ml of dioxane solution, add 1.30 g of selenium dioxide, and react under reflux for 4 hours. After the reaction, dioxane was distilled off, extracted with ethyl acetate and water, the ester layer was washed with water, washed with brine, dried, concentrated, and column chromatographed to obtain ethyl 2-formyl-4-quinolinecarboxylate (intermediate VI), yield 99%. 1 H NMR (CDCl 3 , 400MHz): δ1.52(t, 3H), 4.56(q, 2H), 7.83(t, 1H), 7.91(t, 1H), 8.35(d, 1H), 8.54(s, 1H), 8.90( d, 1H), 10.29(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com