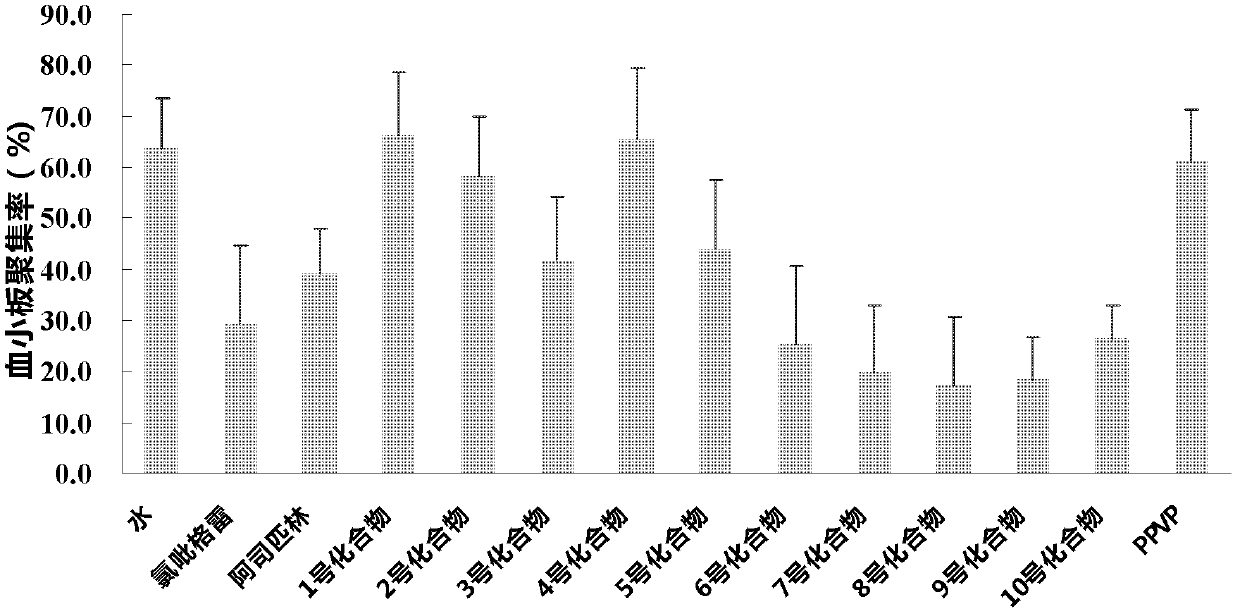
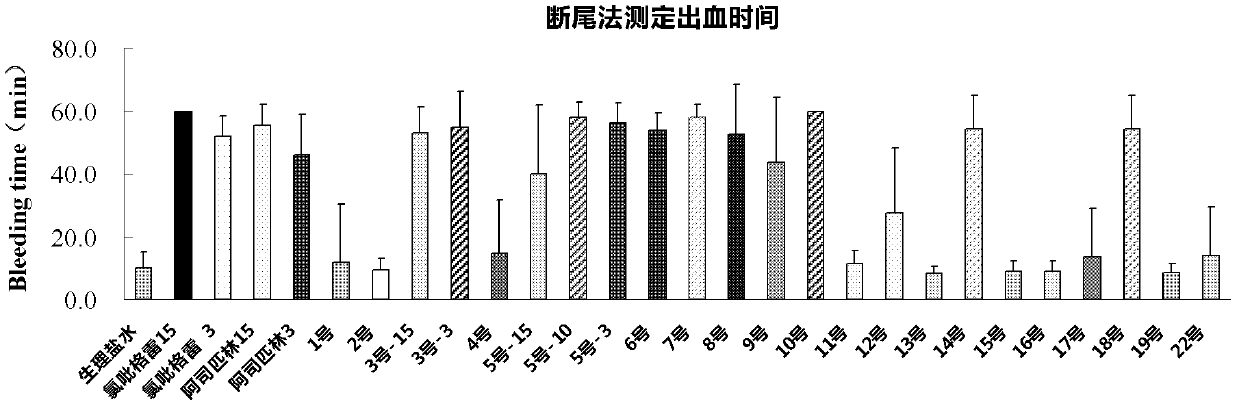
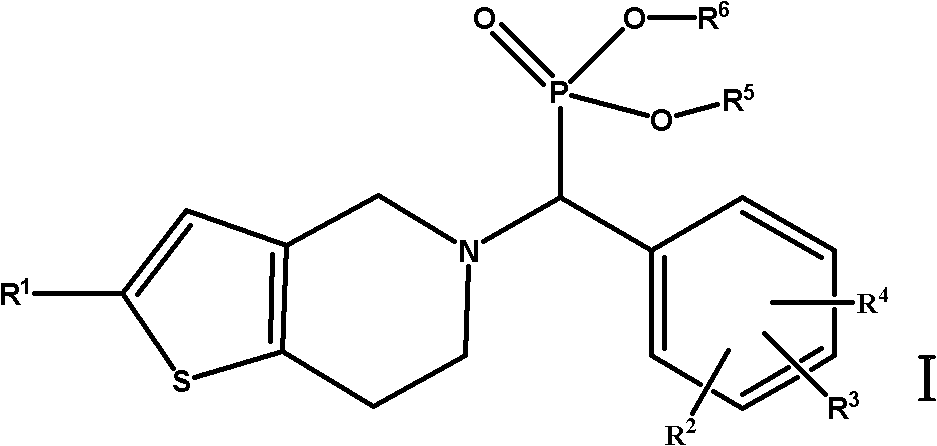
Thienopyridine alpha-amino benzylphosphonate, preparation method and application thereof
A tetrahydrothiophene and hydrocarbon-based technology, applied in the field of medicine and chemical industry, can solve the problems of limited application, limited wide application, and insufficient performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1: α-(2-chlorophenyl)-α-(4,5,6,7-tetrahydrothieno[3,2-c]pyridine Pyridyl) methylphosphonic acid monoethyl ester - preparation of compound 1
[0091]Weigh 6.30g (49.0mmol) of 2-thiopheneethylamine, 8.43g (60.0mmol) of 2-chlorobenzaldehyde and 6.80g (49.0mmol) of diethyl phosphite and add them to 50mL of absolute ethanol to dissolve. React in temperature for 3 days, cool, recover the solvent, add ethanol hydrochloride 17.80mL (58.0g / 500mL, 53.90mmol), paraformaldehyde 1.77g (53.90mmol) and 60mL absolute ethanol, the reaction system is airtight, and the temperature is raised to 60°C, after stirring for one hour, the temperature was raised to reflux for reaction. After 3 days of reaction, the reaction was stopped and column chromatography gave 3.85 g of the product as a white powder with a total yield of 19.3%. mp 188-190°C, 1 H-NMR (CDCl 3 , ppm): δ0.93-0.96 (t, J=7.0Hz, 3H), 3.17-3.30 (m, 4H), 3.52-3.54 (m, 1H), 3.65-3.77 (m, 3H), 4.97-5.01 (d, J=14.85Hz, ...
Embodiment 2
[0092] Example 2: α-(2-fluorophenyl)-α-(4,5,6,7-tetrahydrothieno[3,2-c]pyridine Pyridyl) methyl phosphonic acid monoethyl ester - preparation of compound 2
[0093] According to the method of Example 1, 2-fluorobenzaldehyde was used instead of 2-chlorobenzaldehyde to obtain 3.75 g of the product as a white powder, with a total yield of 19.5%, mp 193-196°C; 1 H-NMR (CDCl 3 , ppm): δ0.87-0.91 (t, J=7.0Hz, 3H), 3.17 (s, 2H), 3.48-3.60 (m, 4H), 4.44 (b s, 1H), 4.77-4.81 (d, J =15.41Hz, 1H), 4.91(b s, 1H), 6.76-6.77(d, J=3.92Hz, 1H), 7.08-7.12(t, J=9.10Hz, 1H), 7.18-7.19(d, J= 5.04Hz, 1H), 7.26-7.30(m, 1H), 7.39-7.48(dd, J=13.72, 6.73Hz, 1H), 8.33-8.36(t, J=6.72Hz, 1H); MS (ESI + , m / z): 356.1 (100%, M+H + ), 245.6 (M-PO(OEt) 2 ) + .
Embodiment 3
[0094] Example 3: α-(3-methoxy-4-hydroxyphenyl)-α-(4,5,6,7-tetrahydrothiophene Preparation of diethyl pheno[3,2-c]pyridyl)methylphosphonate-compound 3
[0095] Weigh 4.00g (26.25mmol) vanillin, 3.48g (25.0mmol) 4,5,6,7-tetrahydrothieno[3,2-c]pyridine, 3.80g (27.5mmol) diethyl phosphite, Add to 70mL of ethanol to dissolve. The reaction was carried out at a bath temperature of 70°C for 3 days, the reaction was stopped, the solvent was recovered, and the product was obtained by column chromatography as 7.50 g of a yellow oily liquid, with a yield of 72.9%; 1 H-NMR (CDCl 3 , ppm): δ1.09-1.11(t, J=7.0Hz, 3H), 1.30-1.33(t, J=7.0Hz, 3H), 2.73-2.87(m, 3H), 3.38-3.41(m, 1H ), 3.71-4.03(m, 8H), 4.18-4.20(m, 2H), 5.94(bs, 1H), 6.68-6.69(d, J=5.04Hz, 1H), 6.90-6.95(m, 2H), 7.03-7.04 (d, J=5.04Hz, 1H), 7.16 (s, 1H); MS (ESI + , m / z): 411.9 (100%, M+H + ), 274.1 (90%, (M-PO(OEt) 2 ) + ). The resulting product was converted into hydrochloride to give a white powder, mp 143-145°C....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com