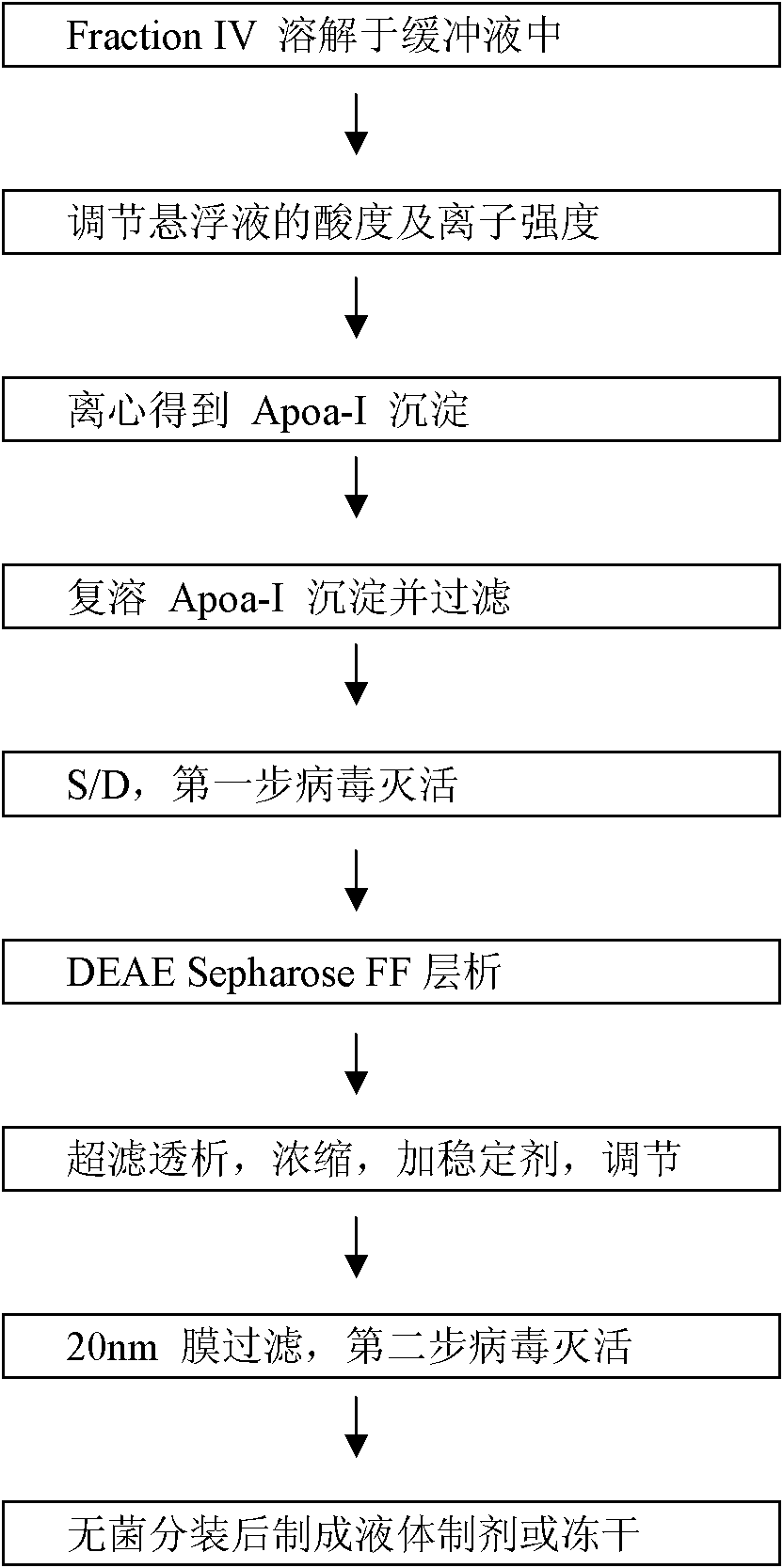
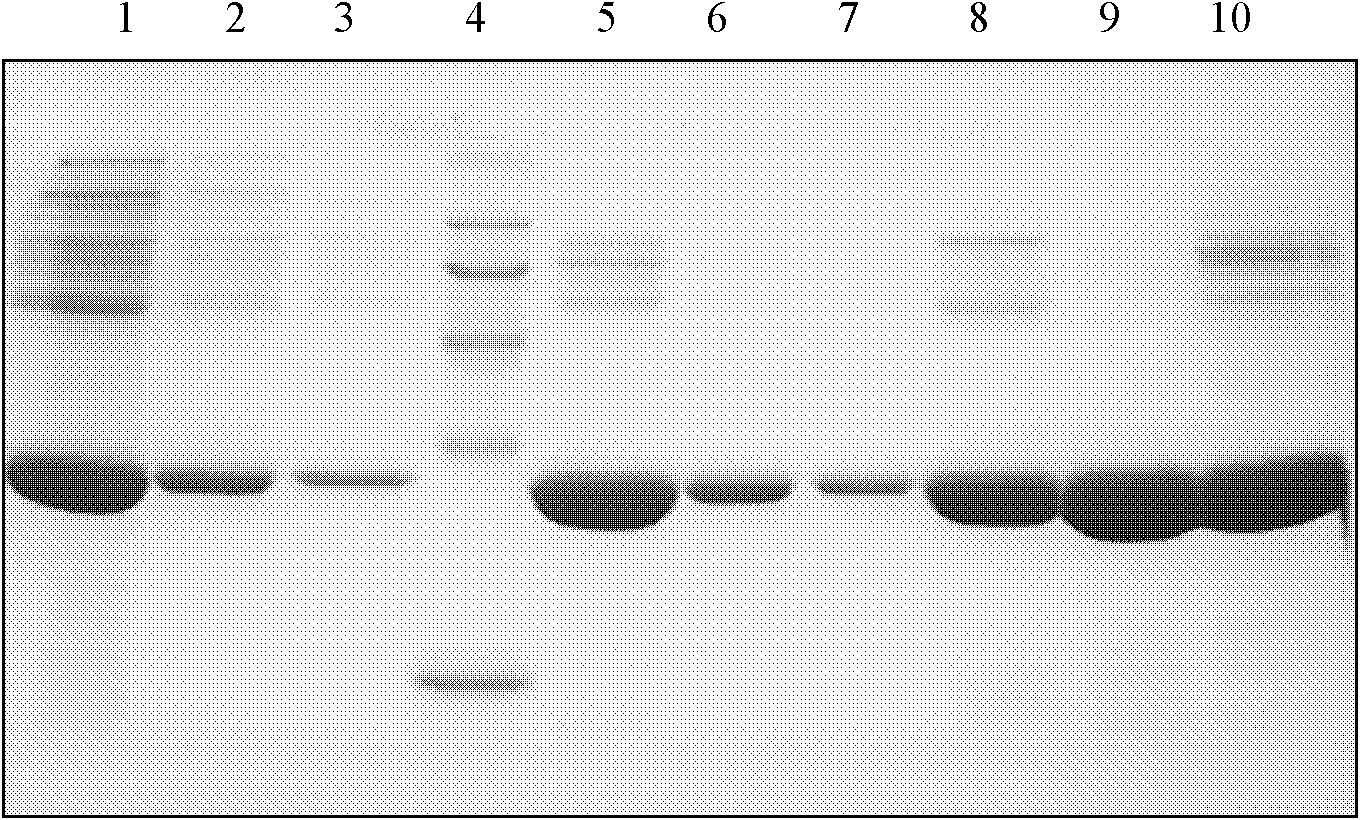
Production technology of high-pure Apoa-I from fourth deposit of human blood plasma component
A technology of component four, human plasma, applied in the field of production technology for the preparation of high-purity apolipoprotein Apoa-I, can solve the problems of low protein yield, loss of Apoa-I, small preparation amount, etc., and achieve high protein recovery rate , Safe and convenient operation, short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, Apoa-I small test sample preparation
[0050] (1) Precipitation, dissolution and pretreatment of component four
[0051] Weigh 50 g of the precipitate of component 4 (wet weight, containing diatomaceous earth), dissolve it in 450 g of acetate buffer solution (0-2°C), adjust the pH value to about 6.0, and stir well to dissolve it.
[0052] (2) centrifugation to obtain Apoa-I precipitate
[0053] Add sodium chloride to the suspension to make the concentration reach about 6%, then adjust the pH value to about 4.8, and cool down to about -3°C. Then use Eppendorf centrifuge 5804R high-speed centrifugation (9000rpm), collect about 40g of the precipitate rich in Apoa-I, and discard the supernatant.
[0054] (3) Redissolve Apoa-I precipitation
[0055] Fully dissolve 40 g of Apoa-I precipitate in 360 g of acetate buffer solution at about 30°C, and then filter with a 0.45 μm filter membrane.
[0056] (4) S / D processing
[0057] Add Tween 80 and TNBP to the filtr...
Embodiment 2
[0069] Embodiment 2, Apoa-I small test sample preparation
[0070] (1) Precipitation, dissolution and pretreatment of component four
[0071] Weigh 50 g of the precipitate of component 4 (wet weight, containing diatomaceous earth), dissolve it in 450 g of tris buffer solution (28-30° C.), adjust the pH to about 10.0, and stir well to dissolve it.
[0072] (2) centrifugation to obtain Apoa-I precipitate
[0073] Add sodium chloride to the suspension to make the concentration reach about 0.2%, then adjust the pH value to about 5.8, and cool down to about 20°C. Then use Eppendorf centrifuge 5804R high-speed centrifugation (9000rpm), collect about 40g of the precipitate rich in Apoa-I, and discard the supernatant.
[0074] (3) Redissolve Apoa-I precipitation
[0075] Fully dissolve 40 g of Apoa-I precipitate in 360 g of tris buffer at about 5°C, and then filter with a 0.45 μm filter membrane.
[0076] (4) S / D processing
[0077] Add Tween 80 and TNBP to the filtrate and incub...
Embodiment 3
[0088] Embodiment 3, Apoa-I small test sample preparation
[0089] (1) Precipitation, dissolution and pretreatment of component four
[0090] Weigh 50 g of the precipitate of component 4 (wet weight, containing diatomaceous earth), dissolve it in 450 g of phosphate buffer (14-16°C), adjust the pH to about 8.5, and stir well to dissolve it.
[0091] (2) centrifugation to obtain Apoa-I precipitate
[0092] Add sodium chloride to the suspension to make the concentration reach about 1%, then adjust the pH value to about 6.50, and cool down to about 0°C. Then use Eppendorf centrifuge 5804R high-speed centrifugation (9000rpm), collect about 40g of the precipitate rich in Apoa-I, and discard the supernatant.
[0093] (3) Redissolve Apoa-I precipitation
[0094] Fully dissolve 40 g of Apoa-I precipitate in 360 g of phosphate buffer at about 15°C, and then filter with a 0.45 μm filter membrane.
[0095] (4) S / D processing
[0096] Add Tween 80 and TNBP to the filtrate and incubate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com