Live bacterial vaccine for controlling intestinal infection caused by clostridium difficile, preparation method thereof and application thereof
A technology for Clostridium difficile and intestinal infection, which is applied to a live bacteria vaccine for preventing and treating intestinal infection caused by Clostridium difficile and its preparation and application fields, and achieves the effects of being convenient for storage, transportation and taking.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Vectors for expressing antigens in vaccines (expression bacteria)
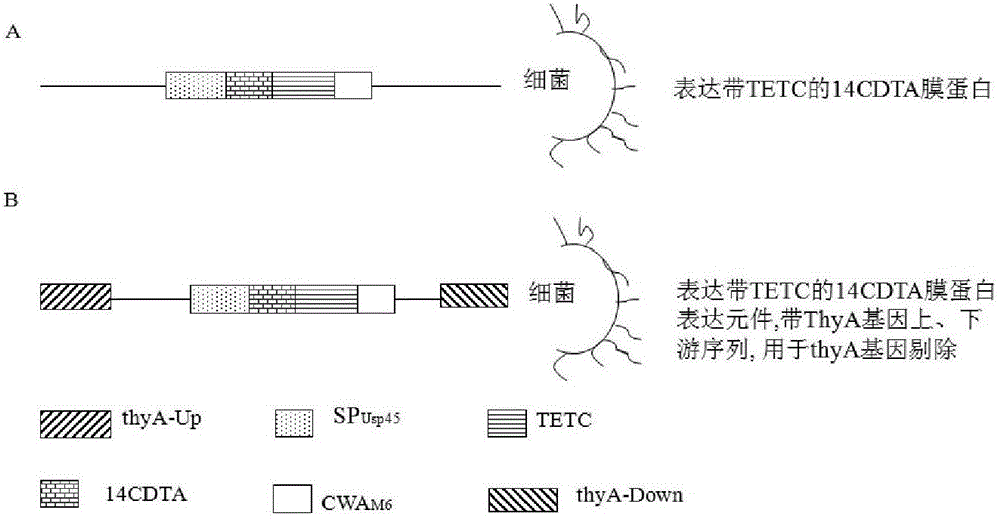
[0057] Lactococcus lactis (Streptococcus lactis; Lactococcus lactis) is used as the carrier to express the 14CDTA protein molecule (antigen) of Clostridium difficile. A kind of bacteria that is harmless to human body and relatively safe (generally regarded as safe, GRAS) is also a kind of food industry bacteria, which is widely used in the dairy industry, such as the lactic acid streptococcus (CICC) preserved in China Industrial Microorganism Culture Collection Center No.: 6023), etc.
[0058] Culture Streptococcus lactis in M17 medium (Difco, Detroit, Mich) or similar medium in an anaerobic or aerobic environment at 30°C. For ThyA gene-deficient Streptococcus lactis, thymidine should be added to the medium (thymidine), the concentration of which is about 20 μM, M17 medium is a medium produced by Difco, and it can be used only after adding distilled water under high pressure.
[0059] 2. Using the ...
Embodiment 2
[0108] Live Recombinant Lactobacillus Vaccine Colonizes Intestines of Golden Hamsters
[0109] In order to observe the recombinant Streptococcus nisin oral vaccine expressing 14CDTA, two recombinants that secrete and express 14CDTA with TETC as a biological adjuvant and membrane-anchored 14CDTA with TETC as a biological adjuvant constructed by the method in Example 1 Streptococcus lactis,) on the prevention and treatment of Clostridium difficile infection, the shuttle plasmid pTRKH2 was used as a carrier to express 14CDTA fusion protein in Streptococcus lactis, and the promoter 59 of Lactococcus lactis subsp.cremoris was used in the experiment (promoter 59, p59) (GeneBank accession No.M24806,) was used as the promoter, and the RBS and signal peptide (75-181, GeneBank accessionNo. M60178) sequence was used as RBS and signal peptide; the terminator of Usp45 gene was selected as the terminator (1514-1553, GeneBank accession No.M60178); 14CDTA expression unit was inserted after th...
Embodiment 3
[0118] Effect of Recombinant Lactobacillus Vaccine on Prevention and Treatment of Clostridium difficile Enteritis
[0119] Lactobacillus LL-PNBCL1003 secreting and expressing 14CDTA with TETC as a biological adjuvant, membrane-anchored Streptococcus lactis LL-PNBCL2003 expressing 14CDTA with TETC as a biological adjuvant, and non-recombinant Lactobacillus (containing a non-recombinant plasmid The construction process of Streptococcus lactis LL-pTRKH2 of pTRKH2 is the same as that in Example 2.
[0120]1. Experimental grouping and immunization methods Take 32 male Syrian golden hamsters (hereinafter referred to as golden hamsters) with a body weight of 100-130 grams, and randomly divide them into 4 experimental groups with 8 animals in each group. The first group (8 animals) is given Normal saline gavage was used as a blank control group, and the second group (8 rats) was given non-recombinant plasmid Streptococcus lactis (LL-pTRKH2) as a positive control ( figure 2 A), the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com