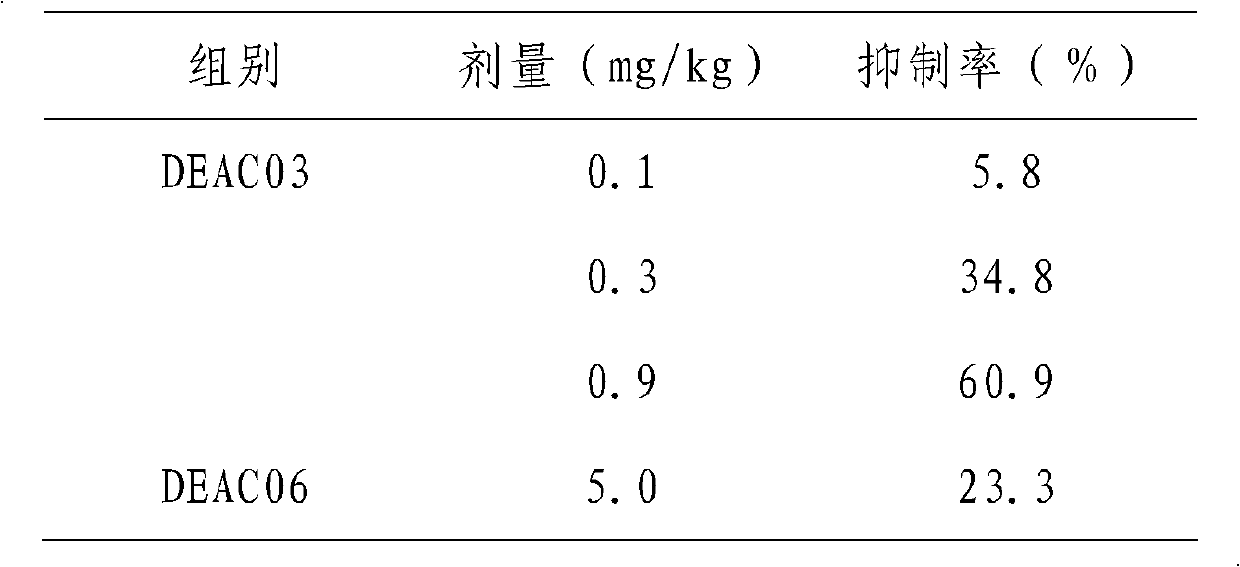
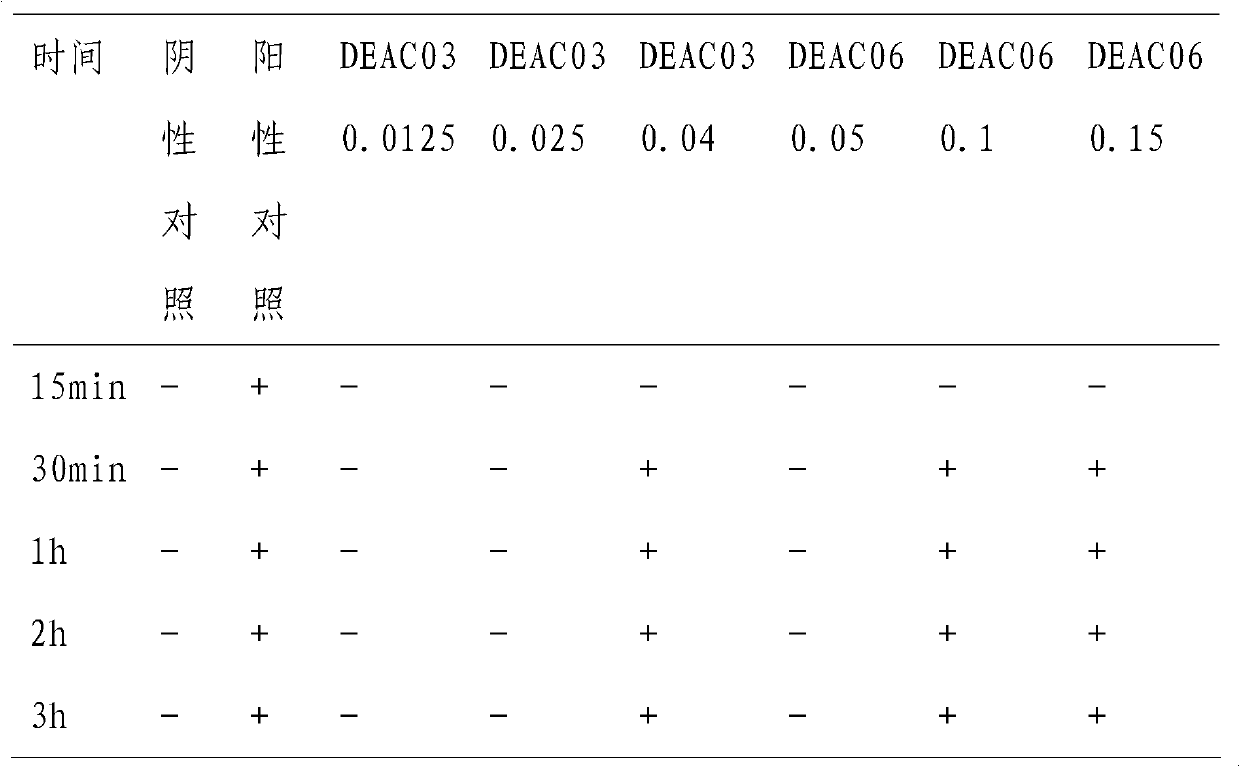
High purity aescine B bulk drug, its preparation method and medical application
A technique for aescin and an API, applied in the field of medicine, can solve the problems of lack of production feasibility, high risk of environmental pollution, low preparation efficiency, etc., and achieves stable and controllable quality, no risk of environmental pollution, and simple processing technology. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
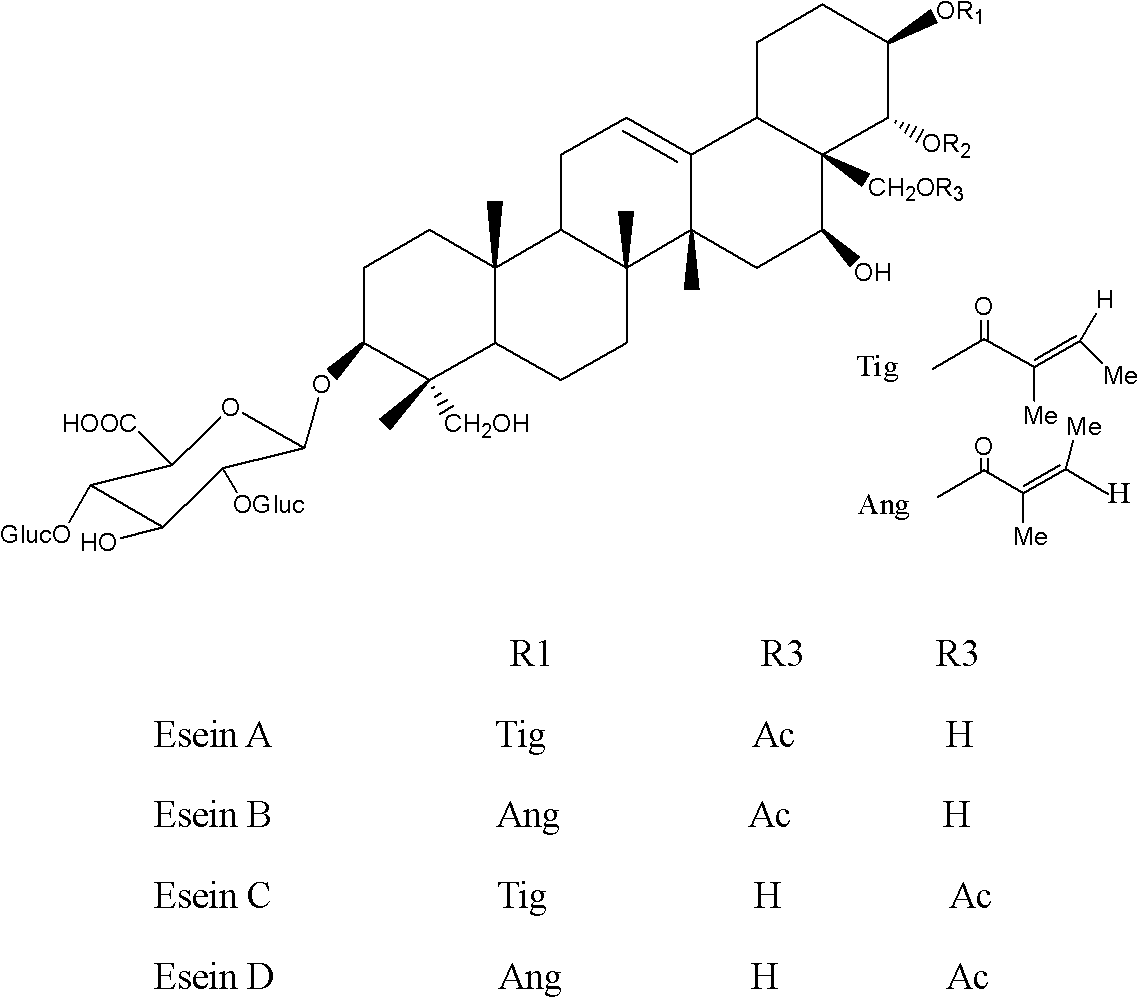
[0026] Aescin total saponins crude product 1g, add distilled water 10ml to dissolve, pass through the chromatographic column equipped with 30g C-18 alkyl bonded phase silica gel (30-50μm), with methanol (30%): 5mM ammonium acetate solution (70%): The mobile phase was eluted with 0.1% acetic acid, and the fractions containing aescin B were collected. Concentrate the fractions under reduced pressure at 40°C, remove all the methanol, dilute with 1 times the volume of the concentrated liquid, and place it at 10°C. A white powder precipitate precipitates, and the precipitate is filtered, washed repeatedly with cold distilled water, and dried to obtain seven Phylloside B (purity 96%).
Embodiment 2
[0028] Aescin total saponin crude product 1g, add distilled water 10ml to dissolve, pass through the chromatographic column equipped with 60gC-18 alkyl bonded phase silica gel (30-50μm), with methanol (35%): 5mM ammonium acetate solution (65%): The mobile phase was eluted with 0.1% acetic acid, and the fractions containing aescin B were collected. Concentrate the fractions under reduced pressure at 45°C, remove all methanol, and place at 4°C to precipitate a white powdery precipitate, which is filtered, washed repeatedly with cold distilled water, and dried to obtain aescin B (purity 98%).
Embodiment 3
[0030] Aescin total saponins crude product 1g, add distilled water 20ml to dissolve, pass through the chromatographic column equipped with 80g C-18 alkyl bonded phase silica gel (30-50μm), with methanol (40%): 5mM ammonium acetate solution (60%): The mobile phase was eluted with 0.5% acetic acid, and the fractions containing aescin B were collected. Concentrate the fractions under reduced pressure at 50°C, remove all methanol, place at 10°C, a white powder precipitate precipitates, filter the precipitate, wash repeatedly with cold distilled water, and dry to obtain aescin B (purity 95%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com