Hydrocarbon catalyzed selective oxidation method
A technology of selective oxidation and hydrocarbons, applied in the field of catalytic application of nanomaterials, it can solve the problems of excessive oxidation reaction, difficult activation, small polarity, etc., and achieve the effect of high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
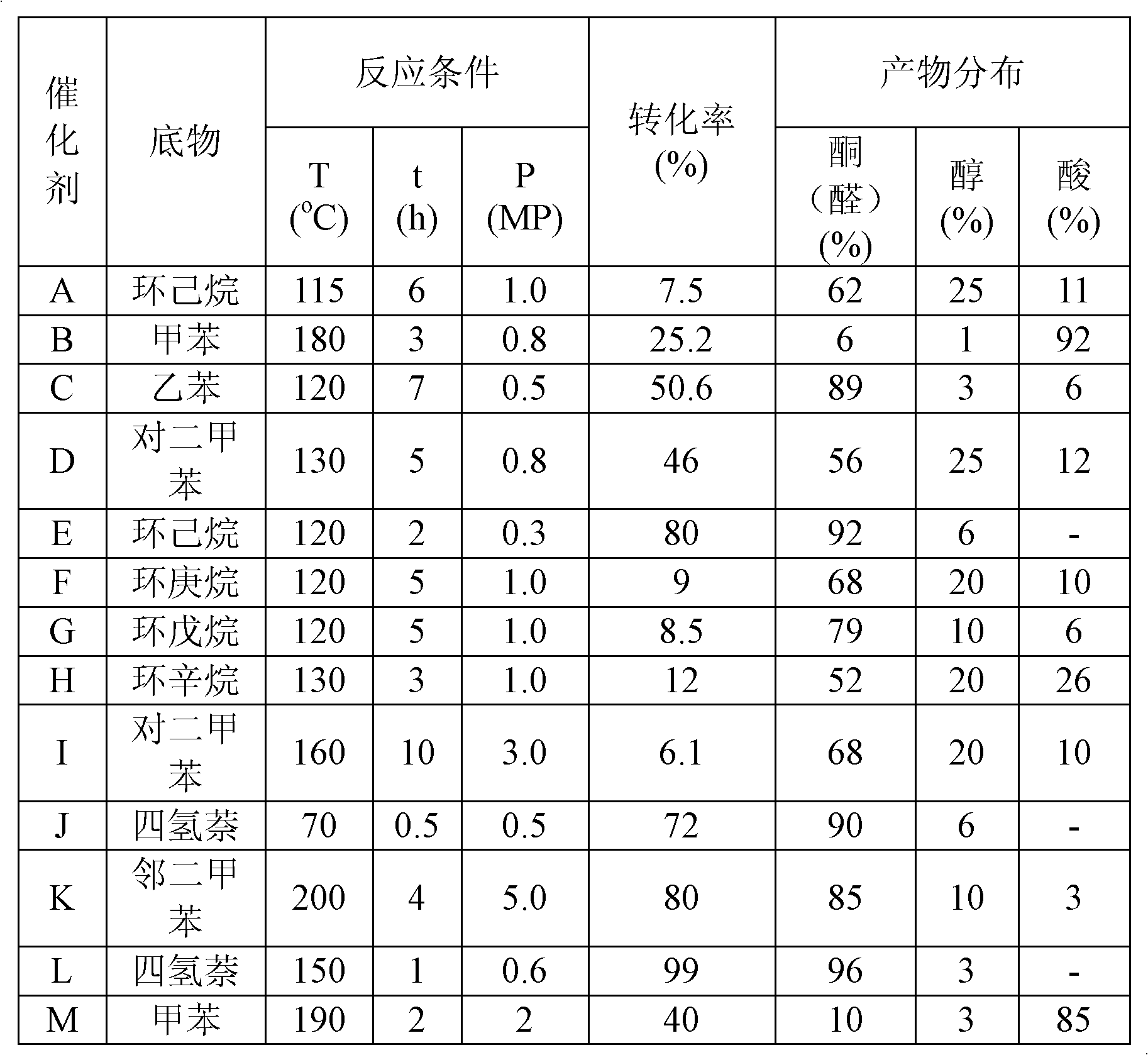
[0025] Embodiment 1 material A (HF-Co-SiO 2 ) preparation
[0026] Mix 20g of Np-10, 25g of cyclohexane and 3g of n-octanol to obtain solution A; dissolve 0.02g of cobalt acetate in 3g of deionized water, and add 0.5g of ammonia water to obtain solution B; 1g of ethyl orthosilicate and 0.2g Mix heptadecafluorodecyltriethoxysilane to obtain solution C; pour solution B into solution A to obtain solution D, add solution C to solution D under vigorous stirring, and age for 8 hours; then, add 10ml of acetone and stir for 30m , centrifuged to obtain a light blue solid; add 30ml of ethanol to this solid, heat and stir for 10m, and centrifuge; repeat this step several times until the surfactant is completely removed; dry at 80°C to obtain the final nanomaterial HF-Co-SiO 2 .
Embodiment 2
[0027] The preparation of embodiment 2 material B-L
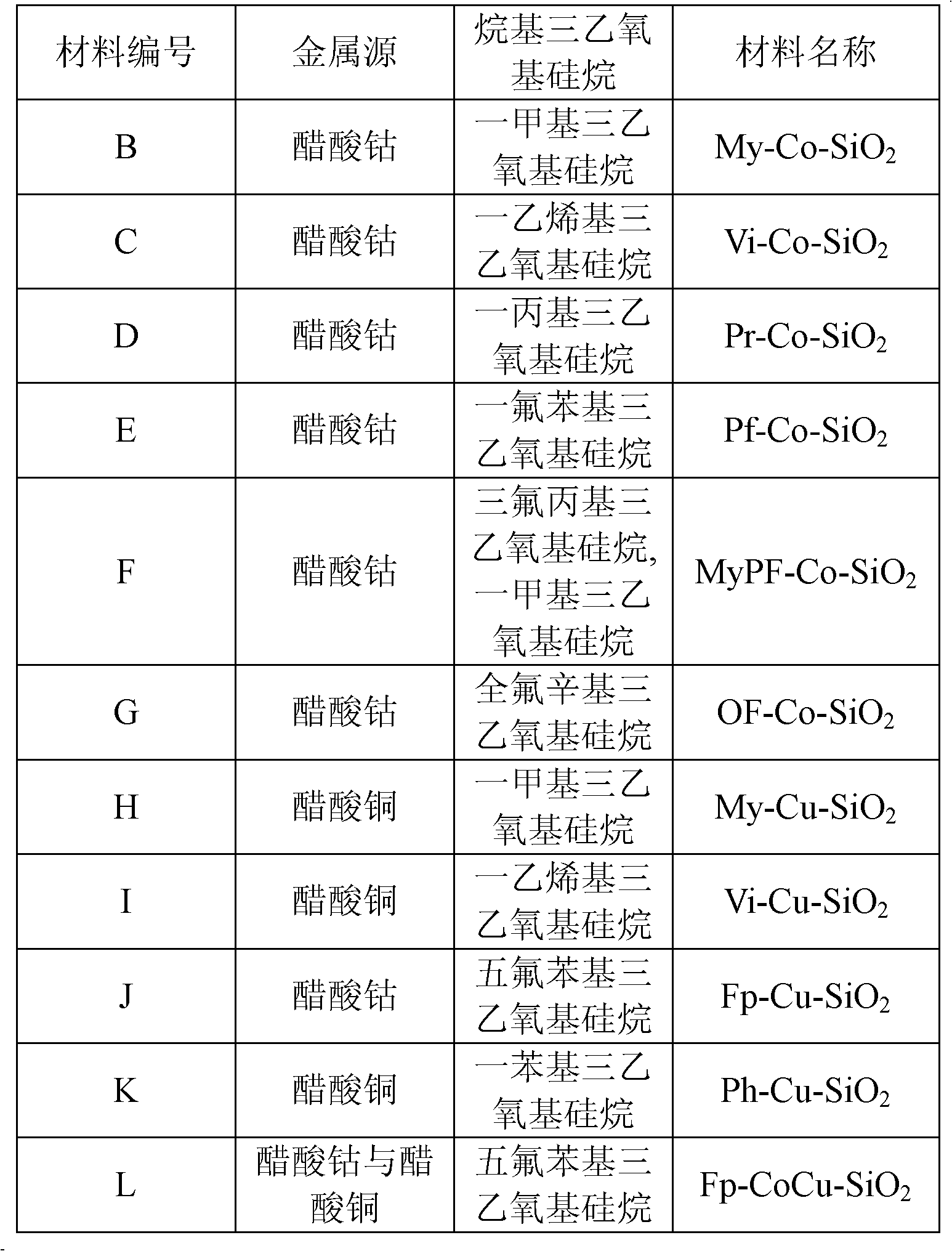
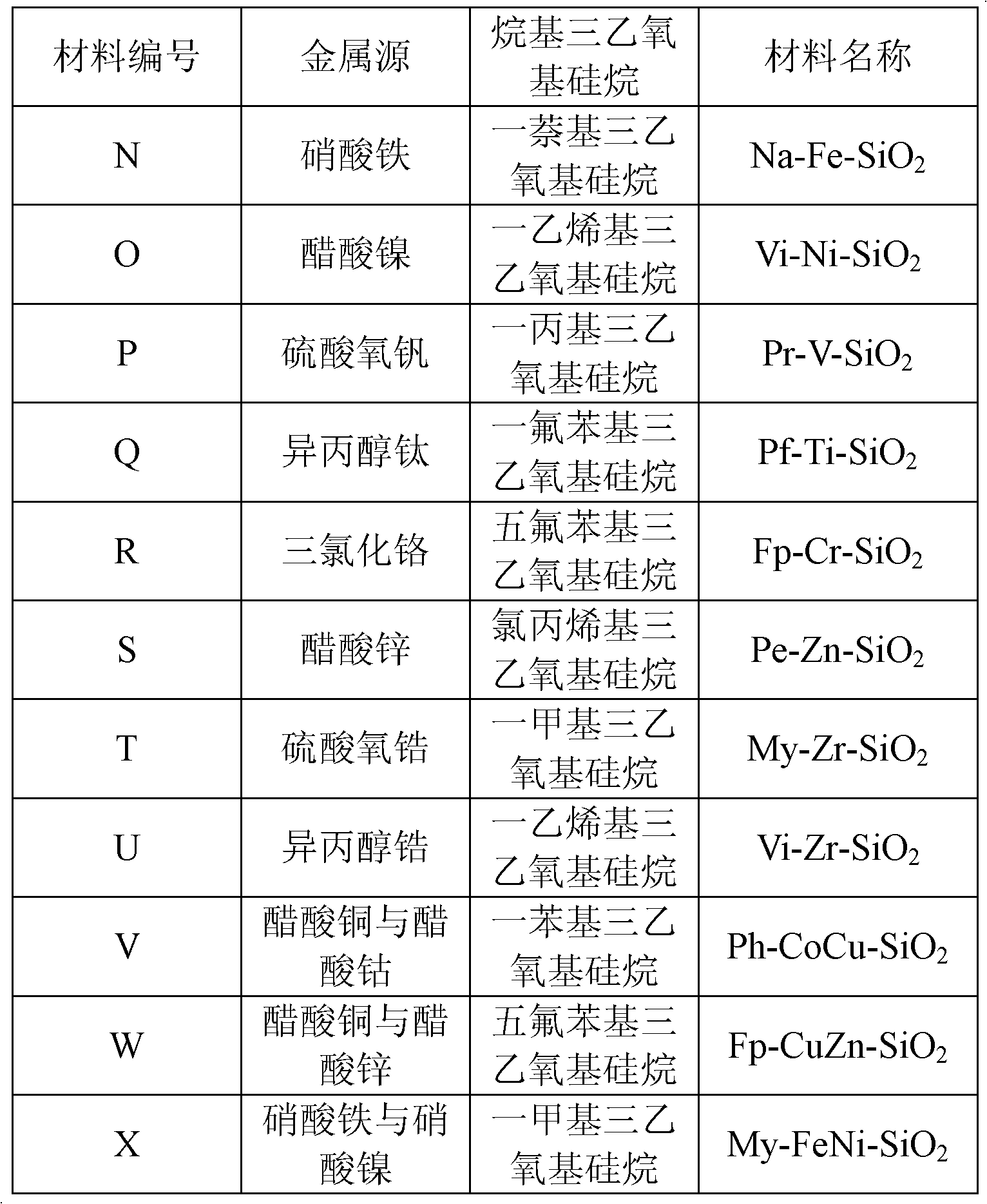
[0028] The preparation method of material B-L is the same as that of material A, the difference lies in the type of metal source or alkyl triethoxysilane, the specific metal source and the type of alkyl triethoxysilane used are shown in Table 1, the obtained material Listed in Table 1.
[0029] The metal source and the type of alkyltriethoxysilane used in the preparation of material B-L in table 1
[0030]
Embodiment 3
[0031] Embodiment 3 material M (Ph-Mn-SiO 2 ) preparation
[0032] Mix 15g sodium dodecylbenzenesulfonate, 35g n-hexane, 6g n-hexanol and 0.5g ammonia water to obtain solution A; dissolve 0.02g manganese acetate in 3g deionized water to obtain solution B; Mix phenyltriethoxysilane to obtain solution C; add solution B and solution C to solution A under stirring conditions, and age for 20 hours; add 15ml of acetone, stir for 20m, and centrifuge to obtain a brown solid; add 60ml of ethanol to the solid , heated and stirred for 15m, centrifuged; repeated this step several times until the surfactant was removed; dried at 100°C to obtain the final nanomaterial Ph-Mn-SiO 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com