Method for modifying vegetable oils and primary plasticizer for vinyl polymers
A technology of vegetable oil modification and vegetable oil, which is applied in the preparation of ester group and hydroxyl reaction, fatty acid esterification, fatty acid oxidation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
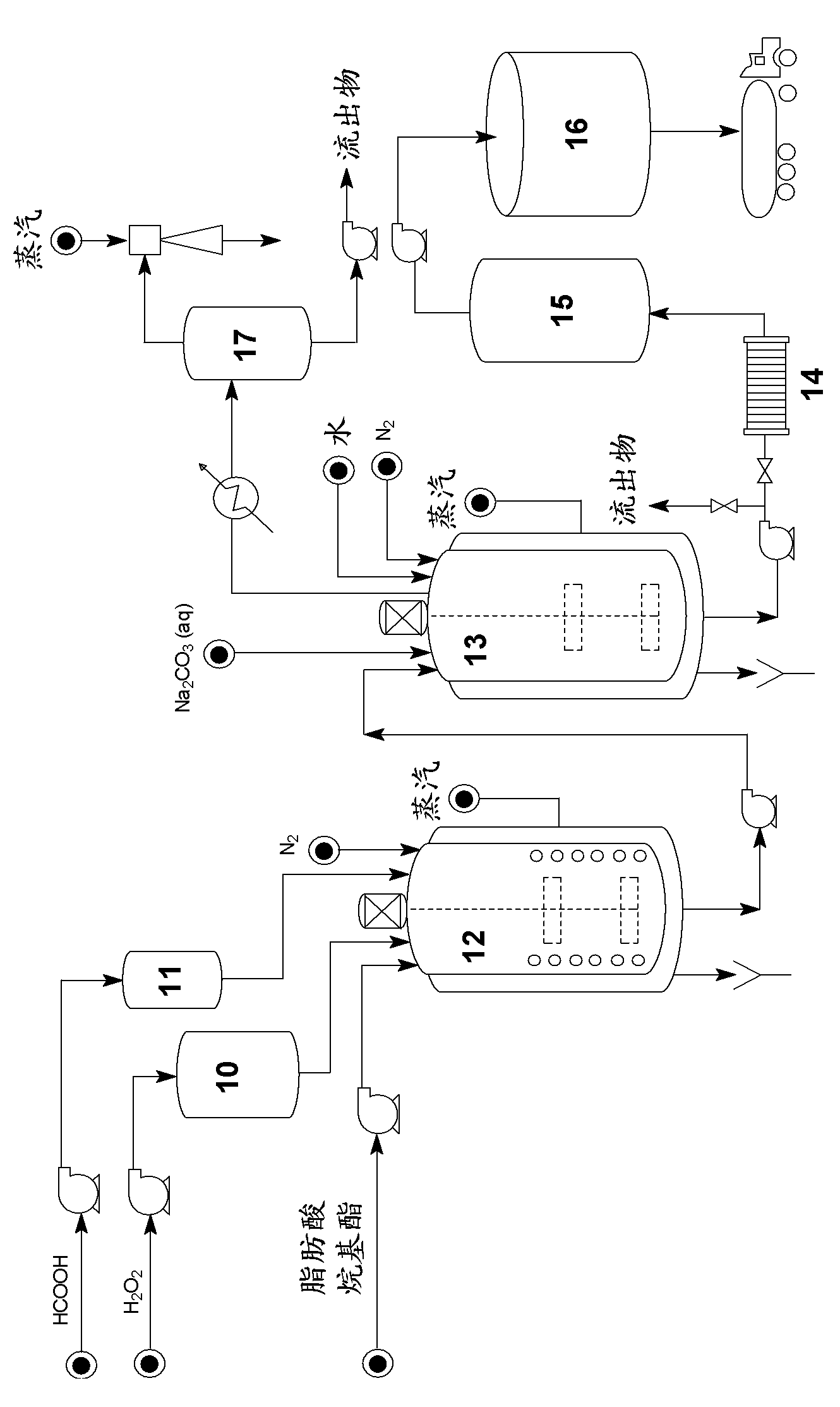
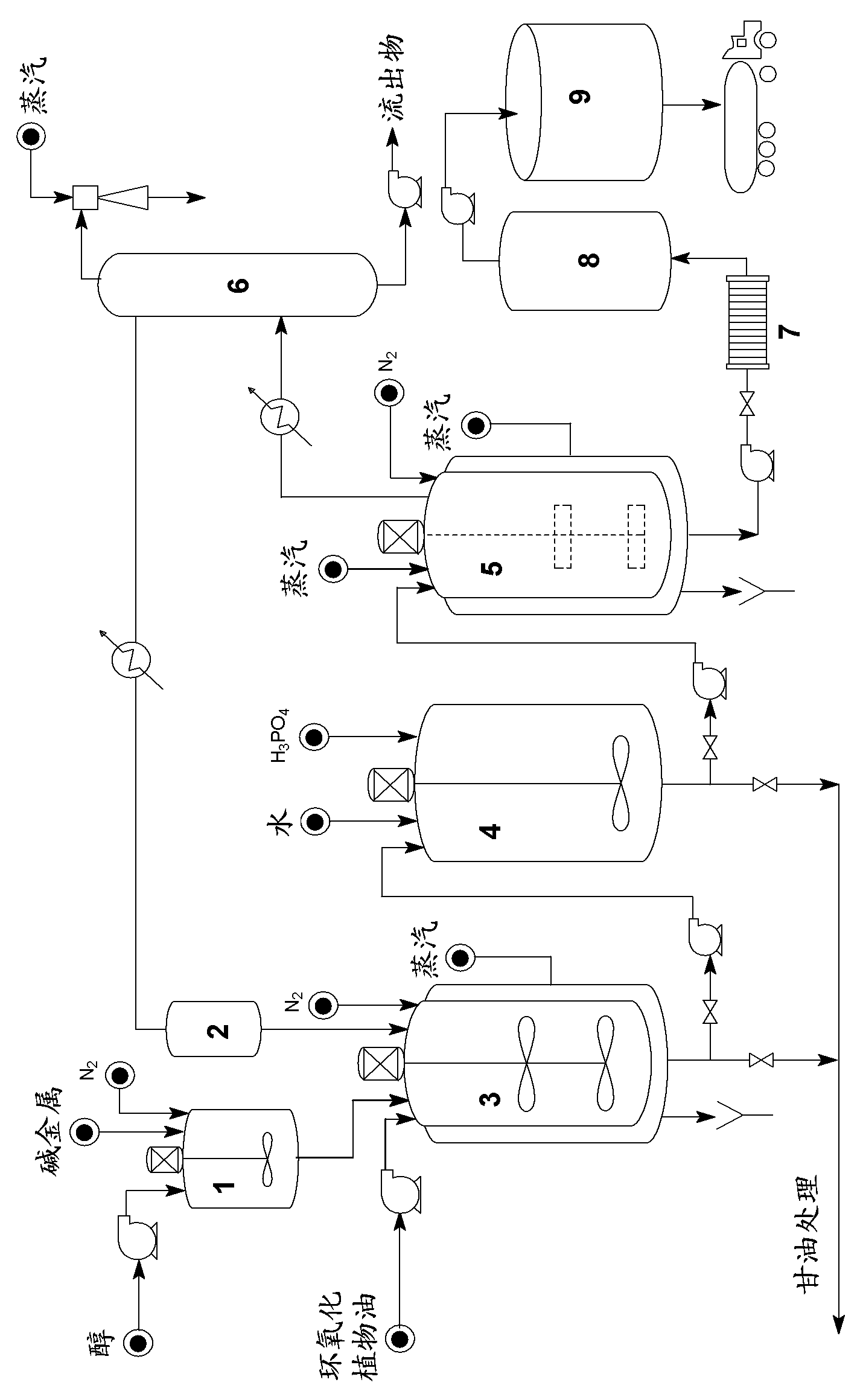
[0061]Transfer about 55 kg of isoamyl alcohol to the catalyst preparation tank. After the system was made inert with nitrogen, 0.7 kg of sodium was added to the alcohol. After the reaction was complete, the catalyst-containing alcohol was transferred to a reactor previously loaded with 100 kg of epoxidized soybean oil (Drapex 6.8; epoxy index = 6.7%) at 70°C. Stirring at 200 rpm, the reaction was kept at 75°C for 2.5 hours. After this period, stirring was discontinued and the system was allowed to stand for 30 minutes. Most of the glycerol produced in the reaction was then drained off and the reaction mixture was transferred to a decanter. Water (approximately 20 L) and phosphoric acid (in an amount sufficient for neutralization) were added and the mixture was stirred for 15 minutes. After standing still for 1 hour, the water containing salt and remaining glycerin was drained off. The mixture containing product and alcohol was transferred to a stripper operated at 150°C un...
Embodiment 2
[0063] About 700 g of isononanol was reacted with 4.8 g of sodium metal under a nitrogen atmosphere. The alkoxide obtained in this way was reacted with 800 g of epoxidized soybean oil (Drapex 6.8; epoxy index = 6.7%) at 90° C. for 2.5 hours. Neutralization, washing and stripping were performed as described in Example 1. After filtration, about 970 g of epoxidized isononyl fatty acid was isolated with the following properties: Acidity Index = 0.95 mg KOH / g; Epoxy Index = 4.8%; Density [20° C.] = 0.923 g.cm -3 ; Chromaticity APHA = 400; Viscosity [20°C] = 47cP.
Embodiment 3
[0065] Under a nitrogen atmosphere, about 800 g of isononanol was reacted with 4.9 g of sodium metal. After the reaction of sodium and alcohol was completed, 800 g of refined linseed oil was added to the resulting alkoxide, and the reaction was kept at 85° C. for 2.5 hours. After separation of the glycerol, the reaction mixture was neutralized, washed and stripped. After filtration, about 1000 g of fatty acid isononyl esters were isolated. About 500 g of this product were subsequently epoxidized with performic acid generated in situ at a double bond:formic acid:hydrogen peroxide molar ratio of 1:0.65:2. The epoxidation was continued at 70° C. for 5 hours, and after washing the reaction mixture with dilute base, followed by drying and filtration, about 520 g of epoxidized isononyl fatty acid esters were obtained with the following properties: acidity index = 0.25 mg KOH / g; epoxy index = 6.15%; density [20°C] = 0.942g.cm -3 ; Chromaticity APHA = 60; Viscosity [20° C.] = 51 c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com