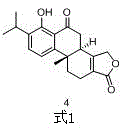
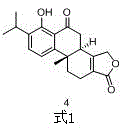
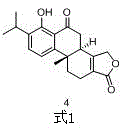
Asymmetric synthesis method of triptolide intermediate
A technology for triptolide alcohol and a synthesis method, which is applied to the production of steroids, bulk chemicals, organic chemistry, etc., can solve the problems of low yield, harsh reaction conditions, insufficient yield, etc., and achieves non-corresponding selectivity Good, mild reaction conditions, the effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: In the synthetic route shown in formula 2 and formula 3, R is phenyl, R 1 is ethyl, R 2 It is trifluoromethyl, and compound 7 is converted into compound 8 under the action of 60% sodium hydride and trifluoromethanesulfonic anhydride. The catalyst is palladium acetate, and the catalyst used for catalytic hydrogenation reduction of compound 3 to compound 4 is Raney Ni.
[0024] Formula 6
[0025] Preparation of compound 7
[0026] In a 250mL three-neck round bottom flask, add 9.5g 1-methyl-5-methoxyl-2-tetralone 5, 6.7g (R)-α-phenylethylamine, 0.95g p-toluenesulfonic acid, and use Dissolve it in 100mL toluene, reflux and separate water under stirring until no water is separated, then cool down to 0 o C, what is obtained is compound 5-1, and 10.0g Nazanov reagent 3-oxo-4-ene-pentanoic acid ethyl ester 6 is slowly added dropwise to the above reaction solution with a constant pressure dropping funnel, after the dropwise addition is completed, the temper...
Embodiment 2
[0041] Embodiment two: in the synthetic route shown in formula 2, R is naphthyl, R 1 is methyl, R 2 For methyl, the chiral amine used is (R)-α-naphthyl ethylamine, compound 7 is converted into compound 8 under the action of potassium hexamethyldisilazide and methanesulfonic anhydride, the reduction of compound 8 The reagent is sodium borohydride-iodine, the catalyst for the carbonyl insertion reaction of compound 9 is palladium chloride, and the catalyst used for catalytic hydrogenation reduction of compound 3 to compound 4 is 5% Pd-C.
[0042] Formula 7
[0043] Preparation of compound 7
[0044] In a 250mL three-neck round bottom flask, add 9.5g 1-methyl-5-methoxy-2-tetralone 5, 8 g (R)-α-naphthylethylamine, 0.95g p-toluenesulfonic acid, and use Dissolve it in 100mL of benzene, reflux and separate water under stirring until no water is separated, and then cool down to 0 oC, to obtain 5-1, slowly drop 10.0g Nazarov reagent 3-oxo-4-ene-pentanoic acid methyl ester 6 ...
Embodiment 3
[0060] In the synthetic route shown in formula 2 and formula 4, R is phenyl, R 1 is isopropyl, R 2 For p-tolyl, the chiral amine used is (R)-α-naphthylethylamine, and the conversion of compound 7 into compound 8 is carried out under the action of potassium hexamethyldisilazide and p-toluenesulfonyl chloride, and the reduction of compound 8 The reducing agent is DiBAl-H, the catalyst for the carbonyl insertion reaction of compound 9 is palladium chloride, and the catalyst used for catalytic hydrogenation reduction of compound 3 to compound 4 is Raney Ni.
[0061]
[0062] Formula 8
[0063] Preparation of compound 7
[0064] In a 250mL three-neck round bottom flask, add 9.5g 1-methyl-5-methoxy-2-tetralone 5, 8g (R)-α-phenylethylamine, 0.95g benzenesulfonic acid, and use 100mL Dissolve it in toluene, reflux and separate water under stirring until no water comes out, and then cool down to 0 o C, to obtain 5-1, slowly drop 10.0g Nazarov reagent 3-oxo-4-ene-valeric acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com