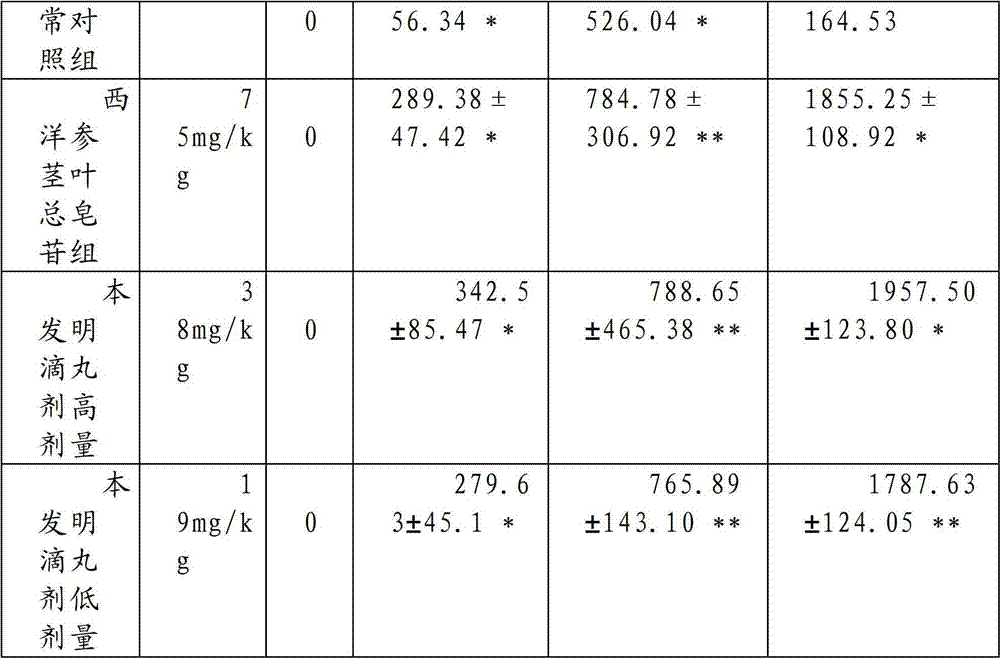Traditional Chinese medicine preparation for treating liver-depression qi-stagnation type viral myocarditis and preparation method thereof
A technology for viral myocarditis and liver stagnation and qi stagnation type, which is applied in the field of traditional Chinese medicine preparations for the treatment of liver stagnation and qi stagnation type viral myocarditis and the field of preparation thereof, so as to achieve the effects of nourishing the myocardium, saving the lives of patients, and protecting heart cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
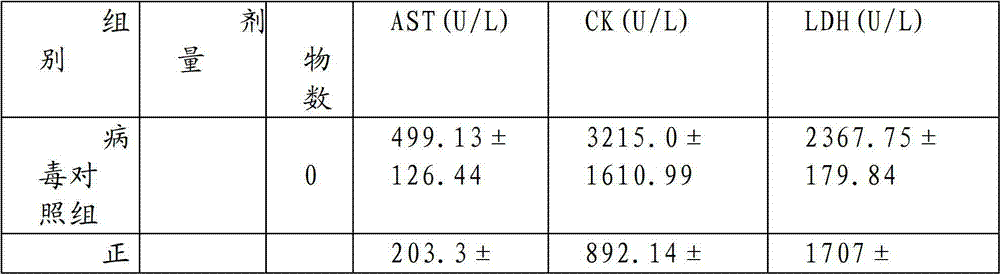
[0054] The preparation method of tablet of the present invention: add Radix Pseudostellariae 1500g, Salvia miltiorrhiza 1500g, Astragalus 1500g, Bupleurum 1500g, Gardenia 1300g, Forsythia 1500g, Rehmannia glutinosa 1500g, Guizhi 1200g, Ligustrum lucidum 1500g, Hedyotis diffusa 1500g, 1500g of Ophiopogon japonicus, 1500g of safflower, 1500g of white peony, 1500g of notoginseng, 1500g of Zhiwu, 1000g of poria, 1500g of loofah, 1500g of chuanxiong, 1500g of curcuma, 1500g of scutellaria, 1500g of Albizia julibrissin, 1500g of silkworm, 1500g of angelica, cypress Kernel 1500g; add 10 times the amount of ethanol, heat and reflux for extraction 3 times, each time for 2 hours, combine the 3 extracts, recover ethanol under reduced pressure and concentrate until the concentration of the medicinal solution is 0.5g crude drug / mL, after suction filtration, the filtrate The relative density is about 1.08 (20°C); the above-mentioned filtrate is eluted with deionized water or distilled water ...
experiment example 1
[0058] Experimental example 1: acute toxicity test of the present invention
[0059] 1. Test materials:
[0060] Animals: Kunming mice, weighing 21-24g, half male and half male, bred by the biological laboratory of Shandong University. Medicine: tablet of the present invention, containing 0.0365g crude drug / sheet
[0061] 2. Method:
[0062] 1. Calculation of LD50: Using the improved Cole's method, the mice were randomly divided into 5 groups, 10 in each group, half male and half male, and the tablet was dissolved in distilled water to make the maximum concentration, and administered according to the maximum allowable volume of the mice. Dosing is followed by 18, 14.4, 11.5, 9.2, 7.4 (g.kg-1) according to the crude drug amount, and after the animal is fasted (can't help water) for 18 hours, it is divided into two administrations (interval half an hour) in one day, 0.5ml each time, observe the animal death.
[0063] 2. Determination of maximum tolerated dose (MTD value): ta...
experiment example 2
[0066] Experimental example 2: Cumulative toxicity test:
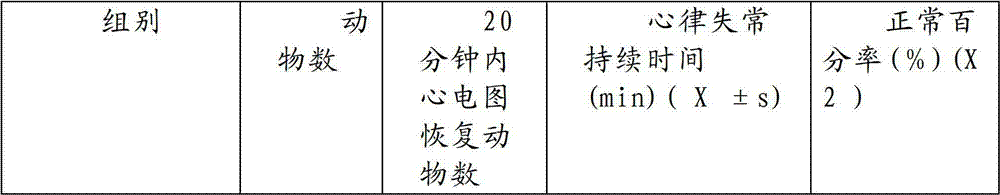
[0067] Traditional Chinese medicine preparation of the present invention is taken orally honey refined pills to mice by 7.69, 19.18 and 43.21g crude drug / kg continuous medication 15 weeks (1.0ml / 100g body weight, every day 2 times) and after drug withdrawal 3 weeks, the result shows: Chinese medicine of the present invention The preparation has no obvious effect on the hair, behavior, defecation, body weight, organ weight, blood picture, liver and kidney function, blood sugar, blood lipid and other indicators of rats. After 1 week and 3 weeks after stopping the drug, there was no significant change in the organs of the rats. It shows that the oral capsule of the traditional Chinese medicine preparation of the present invention has little toxicity to rats after long-term administration, and there is no abnormal reaction after drug withdrawal, so the application is safe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com