Oral calcitonin liposome and lyophilized preparation thereof
A technology of liposome and freeze-drying protective agent, which is applied in the direction of liposome delivery, anti-toxic agent, peptide/protein composition, etc., to achieve good drug effect, increase stability, and strong reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
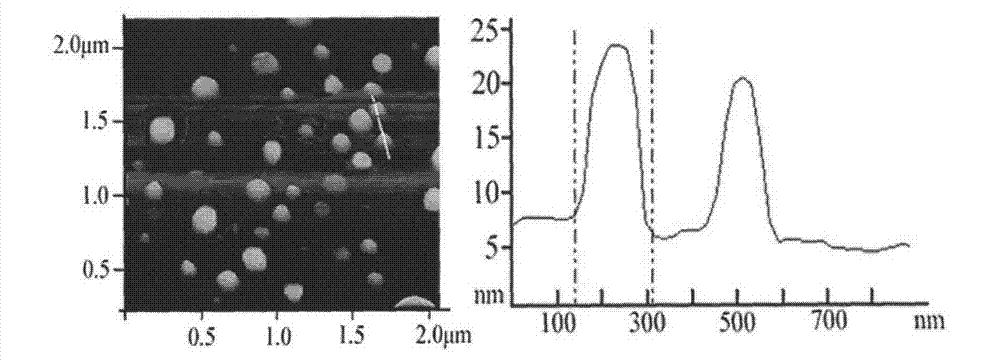
[0030] Dissolve 1.6g of soybean lecithin, 0.4g of DSPG, and 0.3g of cholesterol in a mixed solution of chloroform and methanol, remove the organic solvent by rotary evaporation, put it in a vacuum desiccator overnight, add calcitonin solution, hydrate for 30min, and ultrasonically probe at 400W, 200 Once, after passing through a 450nm microporous membrane for use, the obtained calcitonin liposome was added dropwise to the Arg8 solution, and magnetically stirred to obtain the calcitonin liposome modified by Arg8. The obtained Arg8-modified calcitonin liposomes were added dropwise into the TMC solution, and magnetically stirred to obtain Arg8-modified TMC-coated calcitonin liposomes.
Embodiment 2
[0032] Dissolve 2.0g of soybean lecithin, 0.4g of DSPG, and 0.4g of cholesterol in a mixed solution of chloroform and methanol, remove the organic solvent by rotary evaporation, put it in a vacuum desiccator overnight, add calcitonin solution, hydrate for 30min, and ultrasonically probe at 400W, 400 Once, after passing through a 450nm microporous membrane for use, the obtained calcitonin liposome was added dropwise to the Arg8 solution, and magnetically stirred to obtain the calcitonin liposome modified by Arg8. The obtained Arg8-modified calcitonin liposomes were added dropwise into the TMC solution, and magnetically stirred to obtain Arg8-modified TMC-coated calcitonin liposomes.
Embodiment 3
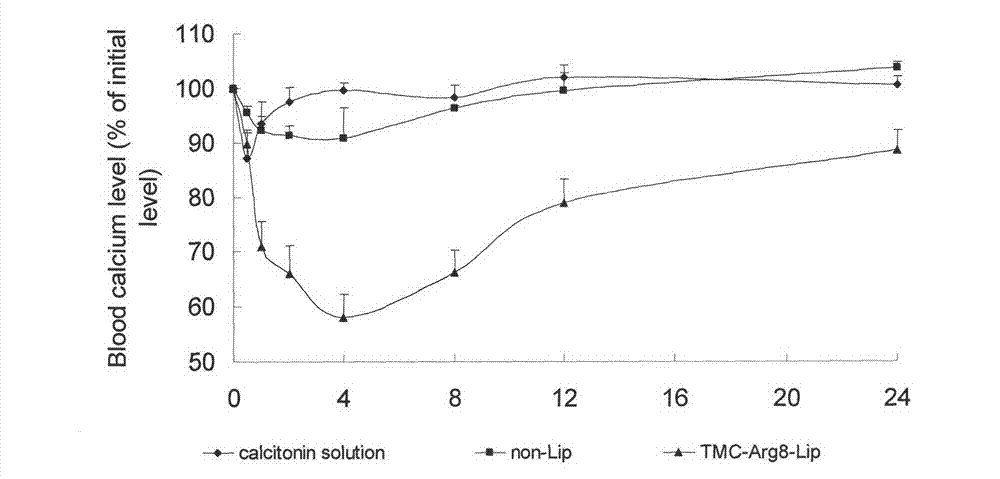
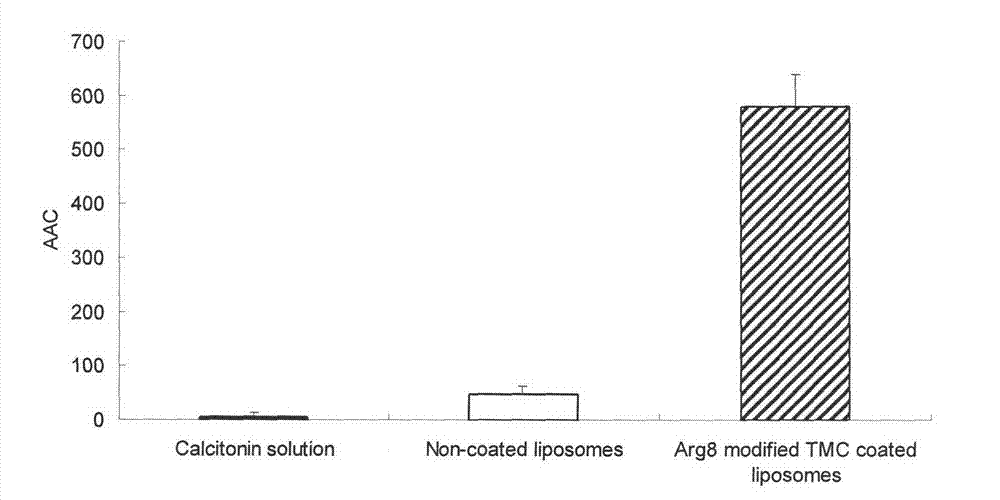
[0034] Dissolve 2.0g of soybean lecithin, 0.5g of DSPG, and 0.3g of cholesterol in a mixed solution of chloroform and methanol, remove the organic solvent by rotary evaporation, put it in a vacuum desiccator overnight, add calcitonin solution, hydrate for 30min, and ultrasonically probe at 200W, 400 Once, after passing through a 450nm microporous membrane for use, the obtained calcitonin liposome was added dropwise to the Arg8 solution, and magnetically stirred to obtain the calcitonin liposome modified by Arg8. The obtained Arg8-modified calcitonin liposomes were added dropwise into the TMC solution, and magnetically stirred to obtain Arg8-modified TMC-coated calcitonin liposomes. The lyoprotectant was added into the preparation, pre-frozen at -70°C for 4 hours, and freeze-dried for 48 hours to obtain Arg8-modified TMC-coated calcitonin liposomes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com