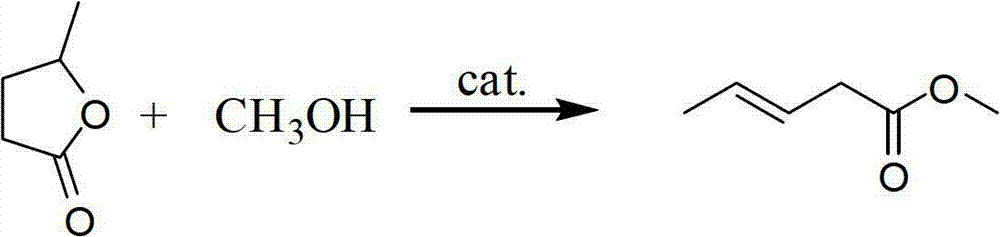
Preparation method of methyl-3-pentenoate
A technology of methyl pentenoate and valerolactone is applied in the field of preparation of unsaturated carboxylic acid esters, can solve problems such as difficulty in isomer separation, avoid expensive price, high yield, and reduce the generation of isomers Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Add a stirring bar, γ-valerolactone (10.01g, 0.1mol) and sulfuric acid (98%, 0.036g, 0.36mmol) into a 25mL two-necked round-bottomed flask, install a rectification column, a nitrogen gas introduction device, and stir in a nitrogen atmosphere Raise the temperature to 170°C, add methanol into the reaction flask through a syringe at a flow rate of 10mL / h, and react for 3.5h. The mixture of methanol and products is distilled from the top of the tower, and the fractions at 70-90°C are collected and analyzed by GC, GC-MS internal standard The content of the target product 3-pentenoic acid methyl ester was determined by the method, which contained 3-pentenoic acid methyl ester 10.84g, the yield was 95%, and the selectivity was 98%.
Embodiment 2
[0063] Add a stirring bar, γ-valerolactone (10.01g, 0.1mol) and trifluoromethanesulfonic acid (98%, 0.055g, 0.36mmol) into a 25mL two-necked round-bottomed flask, install a rectification tower, and a nitrogen gas introduction device. Stir in a nitrogen atmosphere and heat up to 190°C. Methanol is added into the reaction flask through a syringe at a flow rate of 10mL / h. After 5 hours of reaction, the mixture of methanol and products is distilled from the top of the tower. MS internal standard method was used to determine the content of the target product, methyl 3-pentenoate, which contained 10.73 g of methyl 3-pentenoate, with a yield of 94% and a selectivity of 94%.
Embodiment 3
[0065] Add a stirrer, γ-valerolactone (10.01g, 0.1mol) and phosphotungstic acid (0.290g, 0.1mmol) into a 25mL two-necked round bottom flask, install a rectification tower and a nitrogen gas introduction device, stir and raise the temperature in a nitrogen atmosphere To 250°C, add methanol into the reaction flask through a syringe at a flow rate of 10mL / h, react for 8h, the mixture of methanol and product is distilled from the top of the tower, collect the fraction at 70-90°C, and measure it by GC, GC-MS internal standard method The content of target product 3-pentenoic acid methyl ester, wherein contains 3-pentenoic acid methyl ester 9.12g, productive rate 80%, selectivity 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com