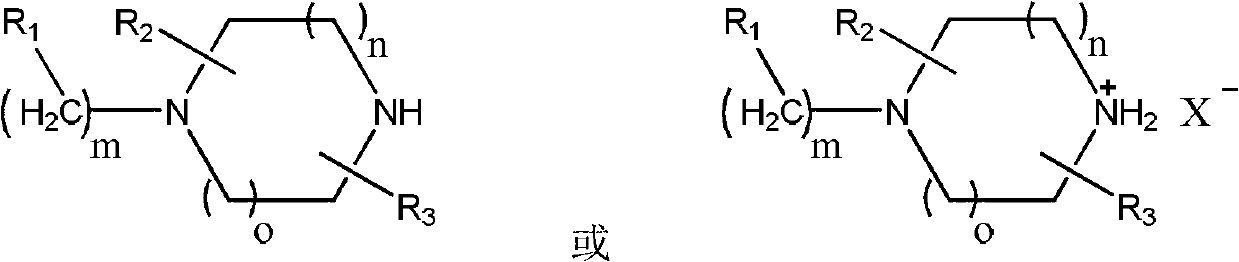
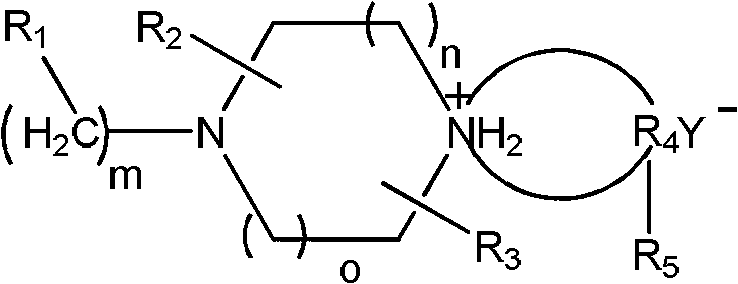
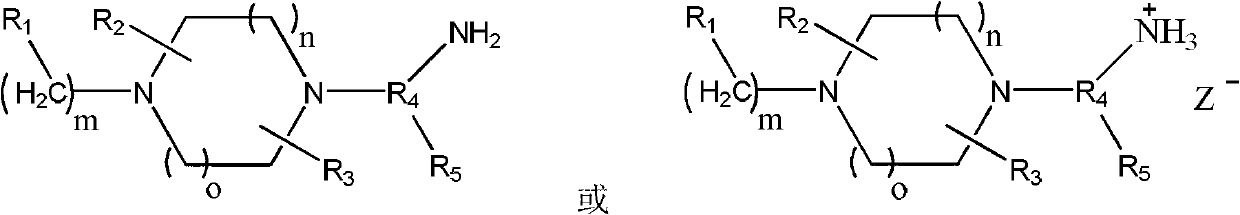
Preparation method of piperazine compound and intermediate thereof
A compound and intermediate technology, applied in the field of drug synthesis, can solve the problems of cumbersome post-processing, low production cost, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] Example 1 Preparation of 2-methoxyphenylpiperazine (SL-1)
[0173]
[0174] Dissolve 32mL (0.44mol) of thionyl chloride in 80mL of chloroform solution, place it in a three-neck flask equipped with a condensing reflux tube and an ice-water bath, stir mechanically, and absorb the tail gas with water. Slowly add a mixed solution of 19.2mL (0.2mol) diethanolamine and 30mL chloroform dropwise with a constant pressure funnel. During the dropwise addition, control the reaction temperature not to exceed 30°C, and the dropwise addition time is 3h. After the dropwise addition, remove the ice-water bath, react at room temperature for 1 hour, then slowly raise the temperature, wait until the solid is completely dissolved and raise the temperature to 60°C, continue the reaction for 0.5 hour, stop heating, cool to room temperature, and suction filter to obtain bis(2-chloroethyl ) amine hydrochloride white powdery solid 34.4g, yield 96.4%, no further purification, directly used in ...
example 2
[0177] The preparation of example 2 4-methoxyphenylpiperazine (SL-2)
[0178]
[0179] Add 5.6mL (0.05mol) of 4-methoxyaniline, 8.925g (0.05mol) of bis(2-chloroethyl)amine hydrochloride, and 10.6g (0.1mol) of sodium carbonate in a 250mL three-necked flask , 1.0g of catalyst and 80mL of distilled water, reflux at 100°C for 24h, after the reaction is complete, after cooling, extract twice with ethyl acetate (2×30mL), combine the organic phases, and wash with 20mL of saturated brine, and wash the organic layer with anhydrous Dry over sodium sulfate, filter, and concentrate under reduced pressure. Add a certain amount of hydrogen chloride ether solution to the crude product, and solids begin to appear. The resulting solids are recrystallized with isopropanol and n-hexane to obtain coffee 4-methoxyphenylpiperazine Hydrochloride color solid 8.9g, the preparation method of 4-methoxyphenylpiperazine is consistent with Example 1, and the total yield (calculated as 4-methoxyaniline) ...
example 3
[0180] The preparation of example 3 phenylpiperazine (SL-3)
[0181]
[0182] The preparation method was the same as in Example 1, and 2-methoxyaniline was replaced by aniline to obtain 12.6 g of phenylpiperazine with a total yield of 77.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com