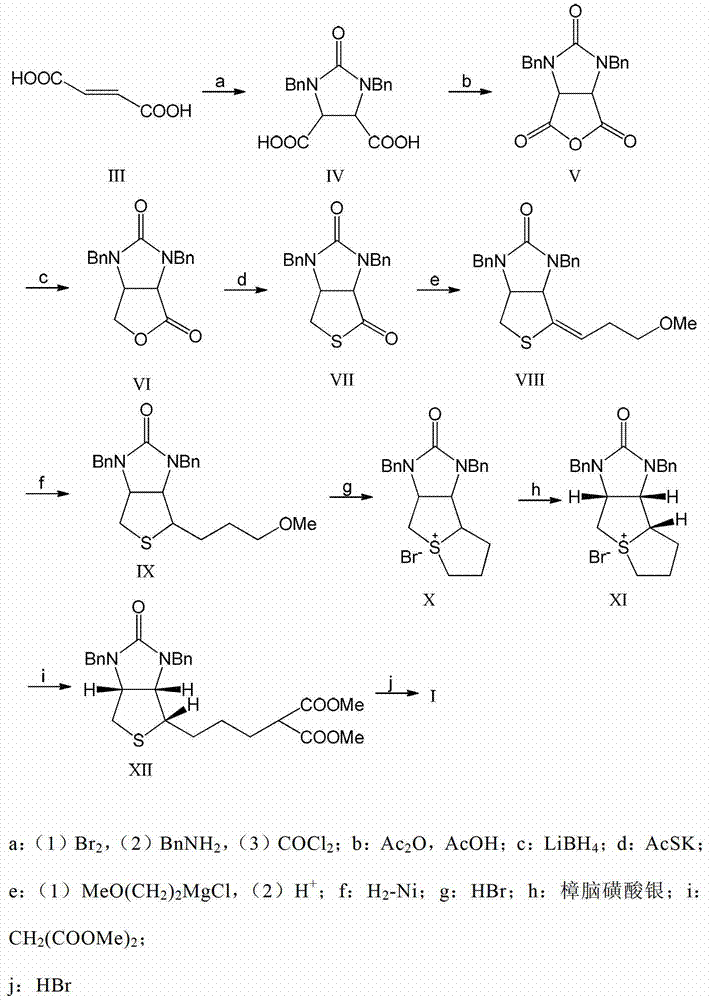
Method for synthesizing d-biotin by catalytic double debenzylation
A biotin and compound technology, applied in the field of chemical synthesis of d-biotin, can solve the problems of complex d-biotin process steps, low yield and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
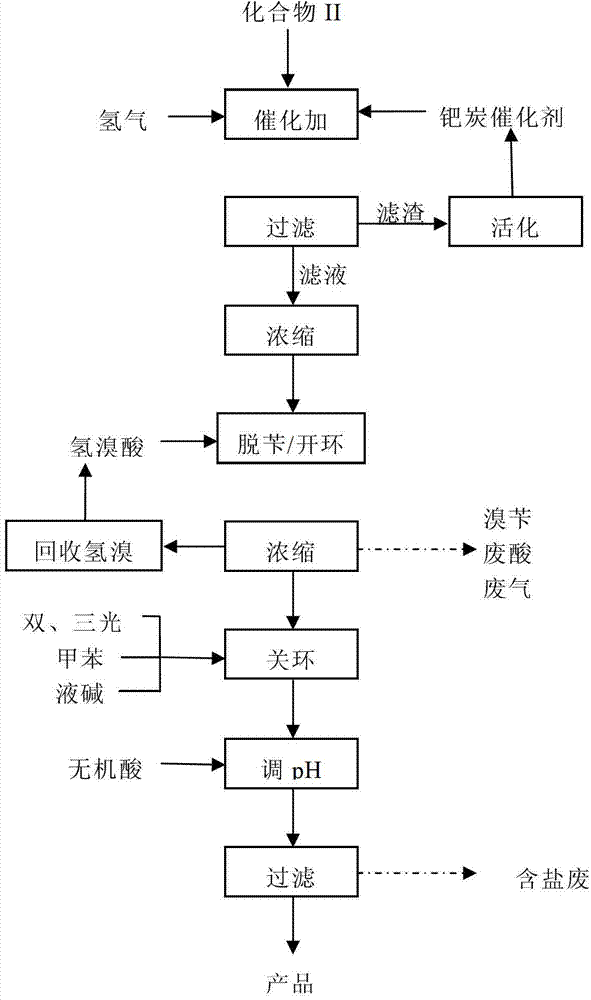
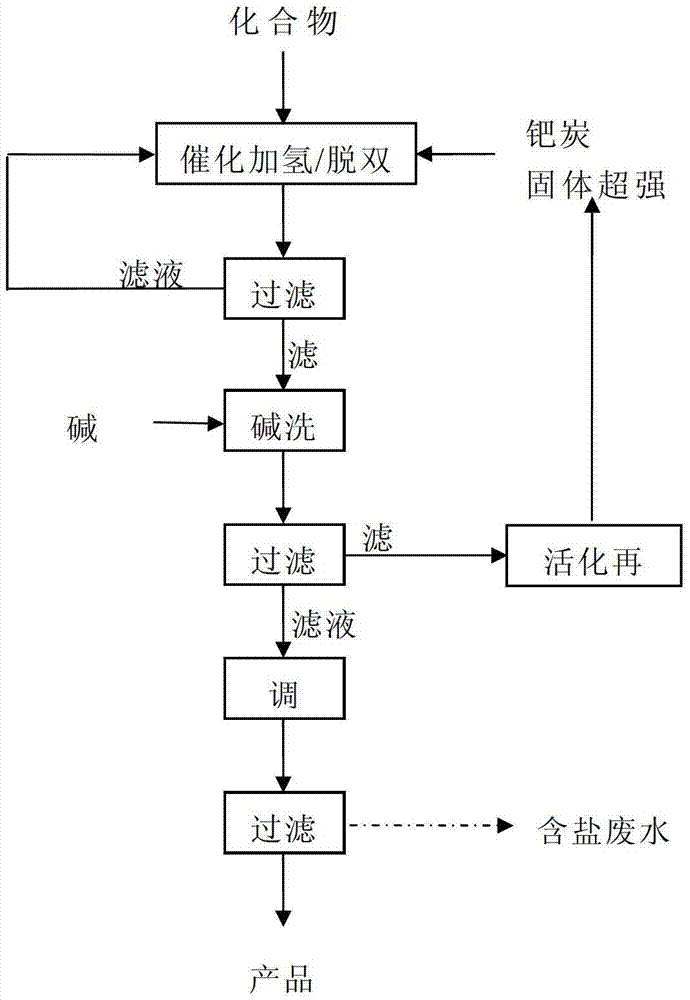
[0075] 30 g of compound II was dissolved in 150 ml of ethanol and added to the autoclave. Then add 10% palladium carbon catalyst 3.0g, SO 4 2- / ZrO 2 -La solid superacid catalyst 1.5g, feed hydrogen until the pressure reaches 5.0MPa, heat to raise the temperature to 120°C, add hydrogen until the pressure is 7.5MPa, start the stirrer, control the rotating speed to 500rpm, and react for 6 hours . After the reaction, cool down and filter. The filtrate is retained and reused in the next production. After the filter residue was rinsed with ethanol, it was added to 50ml of 5% sodium carbonate solution, washed thoroughly, and then filtered. The filter residue is reused in production after washing and activation treatment. The filtrate was adjusted to about 1.0 with 5% sulfuric acid, and allowed to stand for crystallization. After filtering, the filter cake was washed, dried, and weighed to obtain 11.8 g of d-biotin. Converted into a molar yield of about 68%, the tested produc...
Embodiment 2~6
[0077] The steps of Example 1 were repeated, the amount of compound II fixed was 30 g, and other process conditions were changed. The results obtained are shown in Table 1 below.
[0078] Table 1 Embodiment 2~6 result
[0079]
Embodiment 7
[0080] Embodiment 7 Solvent and catalyst recycling experiment
[0081] Repeat Example 1, the process conditions are exactly the same as in Example 1, and the filtrate obtained by filtering after catalytic de-dibenzyl is analyzed for unreacted raw materials and the content of mono- and di-benzyl biotin, and after being converted into the amount of raw materials, add Compound II to 30g, add ethanol to 150ml. After the filter residue after alkali washing was activated, it was added to the high-pressure reactor for the second experiment, which was repeated four times. The results are shown in Table 2.
[0082] Table 2 Circular application experiment results
[0083]
[0084] It can be seen from Table 2 that after the solvent and the catalyst were recycled for 4 times, the performance did not deteriorate significantly, which shows that the method provided by the present invention has industrial practical value.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com