Preparation method and application of chirality azo polyurethane thermal-optic material
A technology of polyurethane and polyurethane prepolymerization, which is applied in the field of chiral azo polyurethane thermo-optical materials and its preparation, can solve the problems of polymer thermal stability reduction and reduction, achieve improved thermal stability, simple preparation process, and high non-toxicity The effect of linear performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
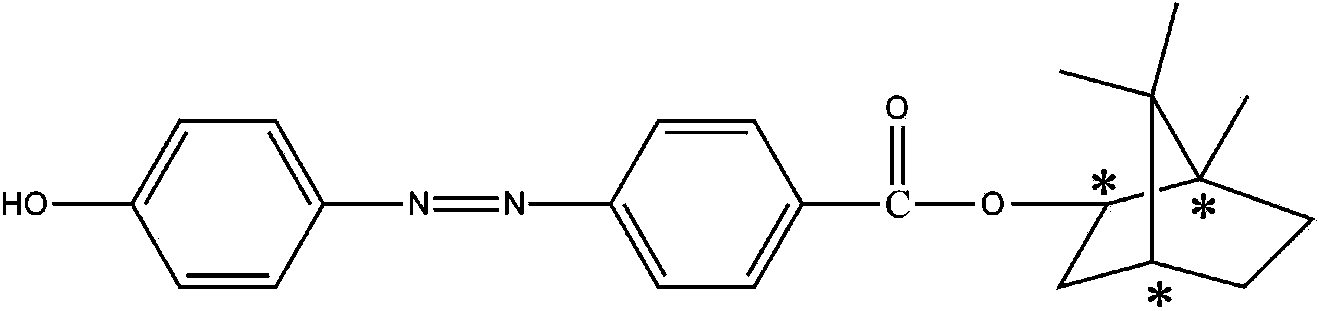
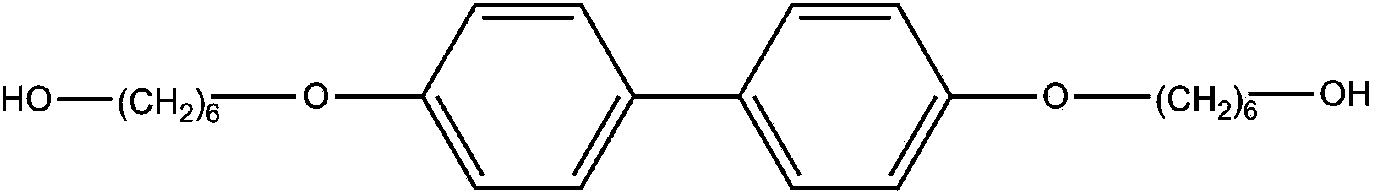
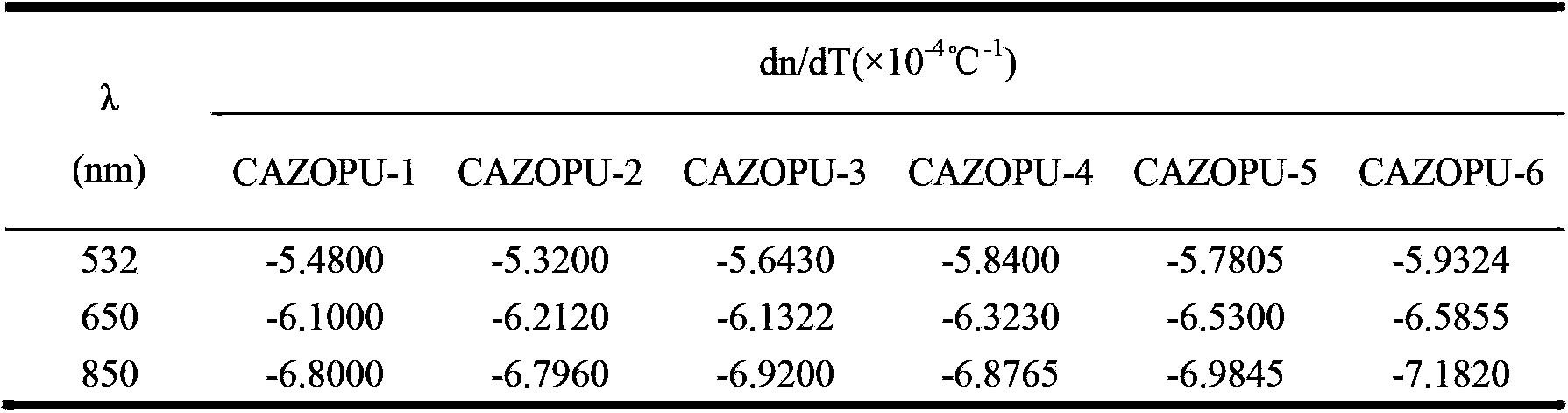
[0027] In a 250mL three-necked flask equipped with a stirring and reflux condenser, 0.66g of 4,4'-bis(6-hydroxyhexyloxy)biphenyl (6BP) was dissolved in 40g of N,N'-diphenyl Methylformamide (DMF), add 0.67g of isophorone diisocyanate (IPDI) and 2 drops of dibutyltin dilaurate (T-12) catalyst to it, react at 90°C for 4h, then add 0.71g of p- Bornyl hydroxyazobenzoate (AZO), continue to react at 90°C for 3h, add 15mL of methanol after the reaction, filter the separated precipitate, wash with 15mL of methanol three times, and vacuum dry at 60°C for 24h to obtain orange-red Chiral azo polyurethane material (CAZOPU-1).
Embodiment 2
[0029] In a 250mL three-necked flask equipped with a stirring and reflux condenser, 1.02g of 4,4'-bis(6-hydroxyhexyloxy)biphenyl (6BP) was dissolved in 65g of N,N'-diphenyl Methylformamide (DMF), add 1.13g of isophorone diisocyanate (IPDI) and 2 drops of dibutyltin dilaurate (T-12) catalyst, react at 90°C for 5h, then add 1.15g of p- Bornyl hydroxyazobenzoate (AZO), continue to react at 90°C for 4h, add 20mL of methanol after the reaction, filter the precipitated precipitate, wash with 20mL of methanol three times, and vacuum dry at 60°C for 30h to obtain orange-red Chiral azo polyurethane material (CAZOPU-2).
Embodiment 3
[0031] In a 250mL three-neck flask equipped with a stirring and reflux condenser, 0.51g of 4,4'-bis(6-hydroxyhexyloxy)biphenyl (6BP) was dissolved in 30g of N,N'-diphenyl Methylformamide (DMF), add 0.53g of isophorone diisocyanate (IPDI) and 2 drops of dibutyltin dilaurate (T-12) catalyst to it, react at 90°C for 4h, then add 0.60g of p- Bornyl hydroxyazobenzoate (AZO), continue to react at 90°C for 3h, add 15mL of methanol after the reaction, filter the precipitated precipitate, wash with 20mL of methanol three times, and vacuum dry at 60°C for 36h to obtain orange-red Chiral azo polyurethane material (CAZOPU-3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com