Polyethylene glycol-polybeta-aminovite segmented copolymer and preparation method thereof
A technology of block copolymer and polyethylene glycol, which is applied in the field of poly-β-urethane, can solve the problems of high cost of poly-β-urethane, few types of secondary amines, and high price, and achieve strong variability and low cost. Low, highly adjustable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The present invention also provides a kind of preparation method of polyethylene glycol-poly β-urethane block copolymer, comprises the following steps:
[0045] The compound with the structure of formula (II), the primary amine and the compound with the structure of formula (III) undergo Michael addition reaction in an organic solvent to obtain polyethylene glycol-polyβ-amine as shown in formula (I) Ester block copolymers;
[0046]
[0047]
[0048] Among them, R 1 Independently selected from C3~C15 alkyl group, substituted C3~C15 alkyl group, C3~C15 alkenyl group, C3~C15 alkyne group, C6~C15 aryl group and C3~C15 heterocyclic group one or more;
[0049] R 2 It is a C2~C10 alkylene group, a substituted C2~C10 alkylene group, a C2~C10 alkenylene group, a C2~C10 alkynylene group or -R 4 -S-S-R 5 -; R 4 C1~C5 alkylene, R 5 A C1~C5 alkylene group;
[0050] R 3 It is hydrogen, C1~C8 alkyl group, C3~C8 alkenyl group or C3~C8 alkyne group;
[0051] n and m are ...
Embodiment 1
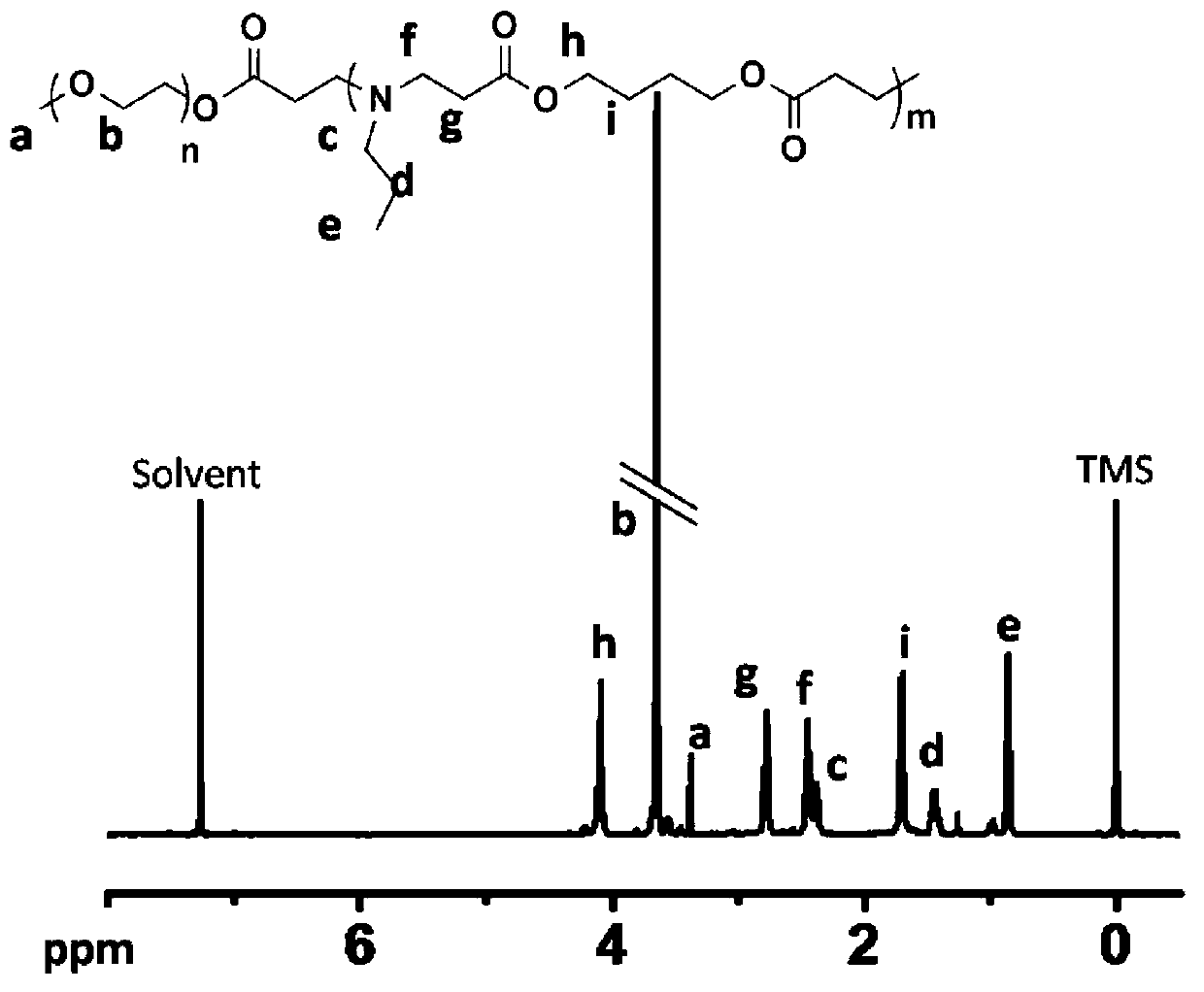
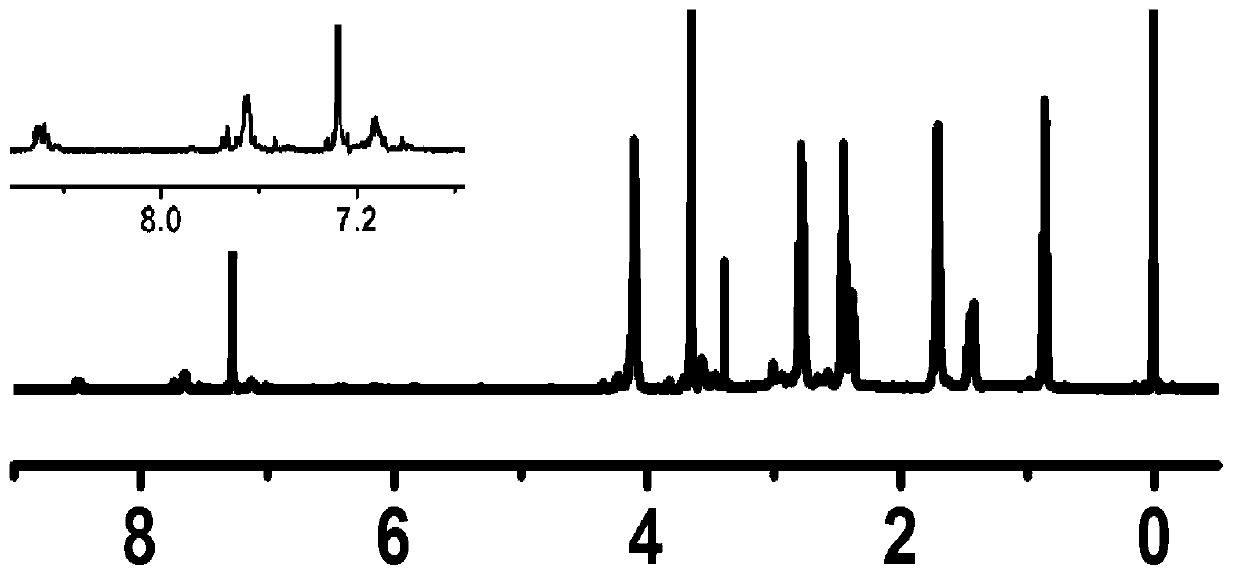
[0061] In a dry reaction bottle, add 0.5mmol of methoxypolyethylene glycol acrylate with a number average molecular weight of 2000Da, 5mmol of n-propylamine, 5mmol of ethylene glycol diacrylate and 5mL of anhydrous chloroform, and seal the reaction at 60°C for 72 hours. After receiving, settle with n-hexane, collect the solid, and dry it under vacuum at room temperature to obtain the polyethylene glycol-poly-β-urethane block copolymer represented by formula (I).
[0062] Using deuterated chloroform as a solvent, the polyethylene glycol-poly β-urethane block copolymer is subjected to nuclear magnetic resonance analysis, and the structure shows that the number average of the polyethylene glycol-poly β-urethane block copolymer is The molecular weight is 4.3×10 3 , where m=10, n=44, R 1 is n-propyl, R 2 is ethyl, R 3 For methyl. The yield of the reaction was 86.9%.
Embodiment 2
[0064] In a dry reaction bottle, add 0.5mmol of methoxypolyethylene glycol acrylate with a number average molecular weight of 2000Da, 5mmol of n-propylamine, 5mmol of propylene glycol diacrylate and 5mL of anhydrous chloroform, and seal the reaction at 60°C for 72 hours. After the reaction is accepted, Settling with n-hexane, collecting the solid, and vacuum drying at room temperature to obtain the polyethylene glycol-poly-β-urethane block copolymer represented by formula (I).
[0065] Using deuterated chloroform as a solvent, the polyethylene glycol-poly β-urethane block copolymer is subjected to nuclear magnetic resonance analysis, and the structure shows that the number average of the polyethylene glycol-poly β-urethane block copolymer is The molecular weight is 4.4×10 3 , where m=10, n=44, R 1 is n-propyl, R 2 is propyl, R 3 For methyl. The yield of the reaction was 83.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com