Application of tri-(2-aminoethyl) amine as carbon dioxide absorbent
A carbon dioxide, aminoethyl technology, applied in the direction of absorption, chemical instruments and methods, separation methods, etc., can solve the problem of not being able to meet the requirements of high-speed, high-capacity, low-cost absorption solvent, slow reaction rate, and relatively expensive price, etc. problems, to achieve the effects of low desorption energy consumption, large absorption capacity, and high recycling rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 :experiment procedure
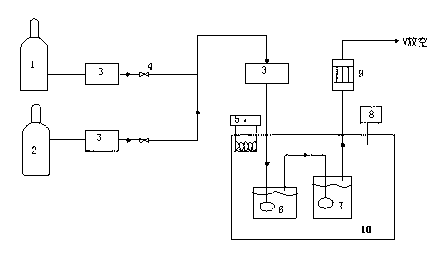
[0023] Devices used such as figure 1 shown. The saturation device and the reaction device are both placed in a constant temperature water tank. Tris(2-aminoethyl)amine is prepared into an aqueous solution of a certain concentration (0.5mol / L-4mol / L) as a carbon dioxide absorbing liquid.
[0024] Specific experimental process: N 2 , CO 2 The gas is mixed by the cylinder through the pressure reducing valve and the mass flow meter in turn, and then enters the saturation device for saturation (the gas is moistened to a certain extent to achieve a certain saturated vapor pressure). The saturated gas enters the reaction device filled with absorption liquid, and then condenses through the condenser and then vents. The temperature of the absorption process is controlled by a temperature controller (20°C-90°C), while the mass flow meter is used to control CO 2 with N 2 The proportion of (that is, to simulate the CO in the absorbed gas ...
Embodiment 2
[0025] Example 2: Absorbent Temperature Investigation
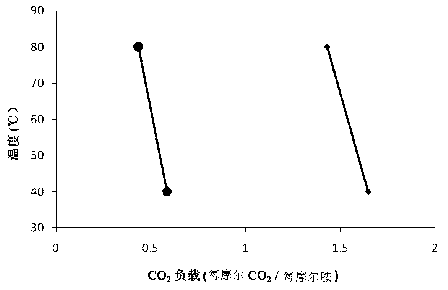
[0026] Using the method described in Example 1, tris(2-aminoethyl)amine is formulated into an aqueous solution with a concentration of 2mol / L as a carbon dioxide absorbing liquid, and under the condition of normal pressure 0.1MPa, the control of CO 2 The volume fraction is 15% (i.e. CO 2 The partial pressure is 15kPa), measure the relationship between the absorption capacity of TAEA and temperature, 8-10 hours to reach absorption equilibrium, and the comparison with the absorption capacity of MEA under the same conditions, such as image 3 shown.
[0027] image 3 is the CO of the aqueous solution of TAEA at different temperatures 2 Absorption capacity and CO of MEA aqueous solution 2 Absorption capacity comparison, ● is MEA, ◆ is TAEA. Depend on image 3 Visible, TAEA as CO 2 As an absorbent, it is feasible at 20°C-90°C, and at different temperatures, it has a larger absorption capacity than MEA. And under...
Embodiment 3
[0028] Example 3: Absorbent Concentration Investigation
[0029] With the method described in Example 1, under the condition of normal pressure 0.1MPa, control CO 2 The volume fraction is 15% (i.e. CO 2 The partial pressure is 15kPa), the temperature of the absorbent is 40°C, and tris(2-aminoethyl)amine aqueous solutions with different molar concentrations are prepared as the carbon dioxide absorption liquid: 0.5 mol / L, 1 mol / L, 2 mol / L, 3 mol / L , 4mol / L to measure the absorption capacity of TAEA. The final results show that the greater the concentration of TAEA in the carbon dioxide absorbing liquid, the CO per unit volume of the absorbing liquid 2 The absorption capacity is increased. Considering economic factors, 1mol / L-3mol / L is more suitable, and 2mol / L is more suitable under the other conditions mentioned above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com