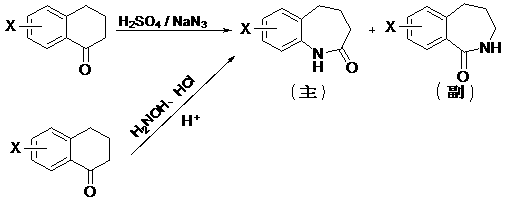
Chemical synthesis method for 2,3,4,5-tetrahydro-1H-2-benzazepin-1-one derivatives
A -1H-2-, chemical synthesis technology, applied in the direction of organic chemistry, can solve the problems of difficult main products, difficult separation, low reaction yield, etc., and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of methyl o-bromobenzoate:
[0026] Add 20.0g o-bromobenzoic acid and 200ml methanol into a 500ml three-necked round bottom flask, stir to dissolve, add SOCl at room temperature 2 17.8g, reacted under reflux for 8h after dropping, concentrated the reaction solution, added 500ml of water, extracted with 500ml of methyl tert-butyl ether, combined the organic phases, and washed with saturated NaHCO 3 The solution was washed, washed with saturated brine, dried and concentrated to obtain 22.5 g of methyl o-bromobenzoate, with a yield of 98%.
[0027] Synthesis of 2-(2'-cyanovinyl) methyl benzoate:
[0028] Add 18.0g of methyl o-bromobenzoate, 8.4g of acrylonitrile, 15.9g of triethylamine, 0.3g of palladium acetate, 170ml of DMF and 0.8g of triphenylphosphine into a 500ml three-necked round-bottomed flask, stir mechanically, and heat Reflux for 10 h, cool to room temperature, add to ice water, extract with petroleum ether, wash the organic phase with 1M hydrochlor...
Embodiment 2
[0032] Synthesis of methyl 2-bromo-6-methoxybenzoate:
[0033] Add 23.1g of 2-bromo-6-methoxybenzoic acid and 200ml of methanol into a 500ml three-necked round-bottomed flask, stir to dissolve, and add SOCl at room temperature 2 17.8g, reacted under reflux for 8h after dropping, concentrated the reaction solution, added 500ml of water, extracted with 500ml of methyl tert-butyl ether, combined the organic phases, and washed with saturated NaHCO 3 The solution was washed, washed with saturated brine, dried and concentrated to obtain 23.5 g of methyl 2-bromo-6-methoxybenzoate, with a yield of 96%.
[0034] Synthesis of 2-(2'-cyanovinyl)-6-methoxybenzoic acid methyl ester:
[0035] Add 20.0g of methyl 2-bromo-6-methoxybenzoate, 8.4g of acrylonitrile, 15.9g of triethylamine, 0.3g of palladium acetate, 170ml of DMF and 0.8g of triphenylphosphine into a 500ml three-hole round bottom In the flask, stir mechanically, heat and reflux for 10h, cool to room temperature, add to ice water...
Embodiment 3
[0039] Synthesis of methyl 2-bromo-7-methoxybenzoate:
[0040] Add 23.1g of 2-bromo-7-methoxybenzoic acid and 200ml of methanol into a 500ml three-necked round-bottomed flask, stir to dissolve, and add SOCl at room temperature 2 17.8g, after dripping, react under reflux for 8h, concentrate the reaction solution, add 500ml of water, extract with 500ml of methyl tert-butyl ether, combine the organic phases, and wash with saturated NaHCO 3 The solution was washed, washed with saturated brine, dried and concentrated to obtain 23.2 g of methyl 2-bromo-7-methoxybenzoate, with a yield of 95%.
[0041] Synthesis of 2-(2'-cyanovinyl)-7-methoxybenzoic acid methyl ester:
[0042] Add 20.0g of methyl 2-bromo-7-methoxybenzoate, 8.4g of acrylonitrile, 15.9g of triethylamine, 0.3g of palladium acetate, 170ml of DMF and 0.8g of triphenylphosphine into a 500ml three-hole round bottom In the flask, stir mechanically, heat and reflux for 10h, cool to room temperature, add to ice water, extract w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com