Halamine antibacterial agent, its preparation method and application
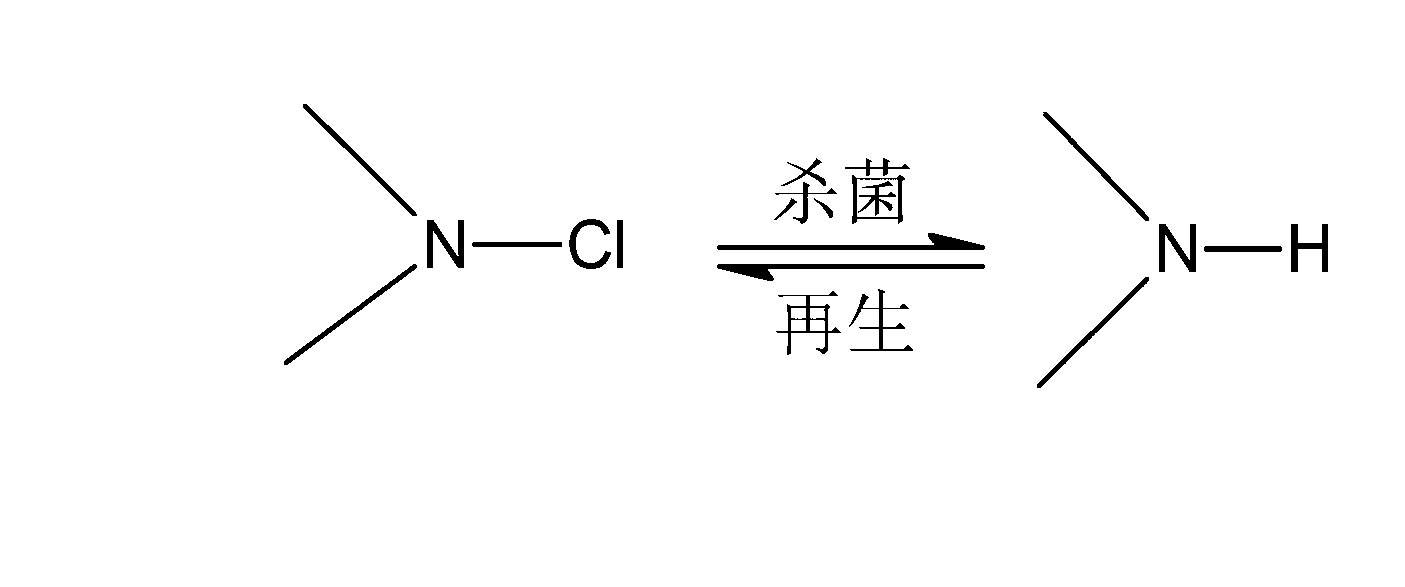
A technology of antibacterial agent and halamine, which is applied in the synthesis and antibacterial field of halamine compounds, can solve the problems of harsh reaction conditions, life-threatening, cumbersome preparation steps, etc., achieve small mechanical strength damage, good antibacterial performance, and simple process operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
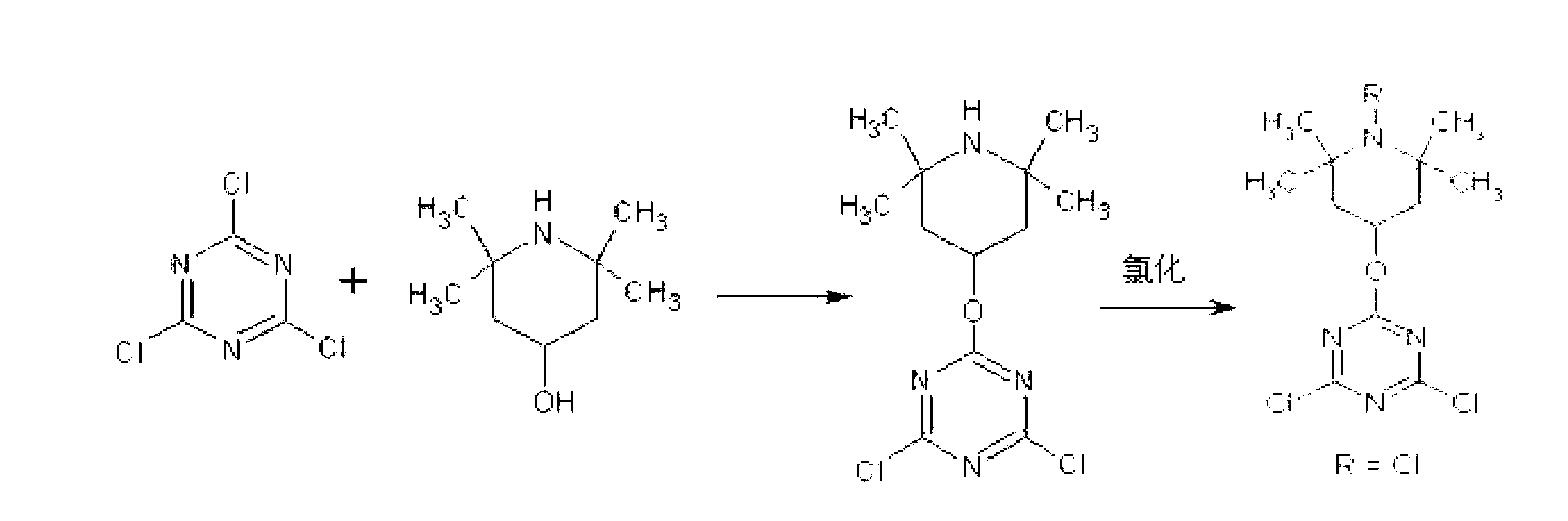
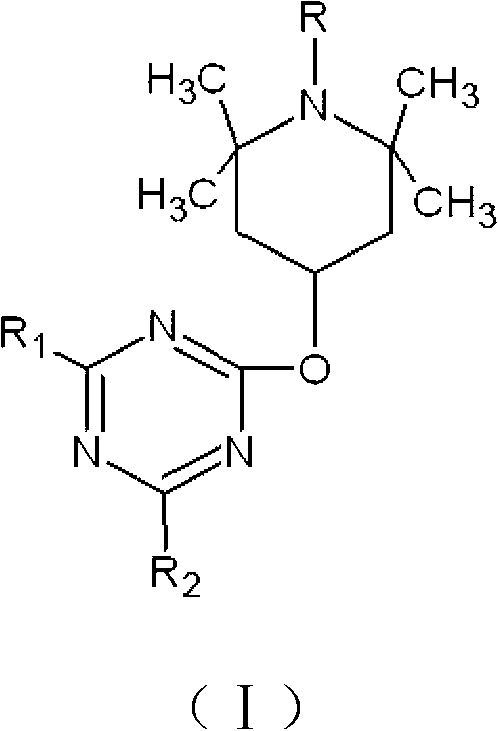
[0044] Preparation of halamine antibacterial agent 2,4-dichloro-6-(1-chloro-2,2,6,6-tetramethylpiperidin-4-oxyl)-1,3,5-triazine
[0045] Weigh 9.22g of cyanuric chloride, dissolve it in 100mL of acetone, weigh 7.86g of 2,2,6,6-tetramethyl-4-piperidinol, dissolve it in 100mL of water, mix the two in Place a 500mL round-bottomed flask in an ice bath and stir well, add 2g of sodium hydroxide, and react at 0°C for 1 hour. After the reaction is completed, filter according to the conventional operation method, and wash with distilled water and acetone until the pH value of the filtrate is 7.0. Dry the obtained solid at 60°C and 0.2MPa in a vacuum oven for 12 hours to obtain the precursor of haloamine antibacterial agent 2,4-dichloro-6-(2,2,6,6-tetramethylpiperidine- 4-oxy)-1,3,5-triazine, and finally dissolve the precursor in a sodium hypochlorite solution with a concentration of 0.1% by mass, and let it stand at room temperature for 1 hour. After the reaction is completed, filter a...
Embodiment 2
[0047] Haloamine antibacterial agent 2-chloro-4-methoxy-6-(1-chloro-2,2,6,6-tetramethylpiperidin-4-oxyl)-1,3,5-triazine preparation of
[0048] Weigh 9.01g of cyanuric chloride derivative 2,4-dichloro-6-methoxyl-1,3,5-triazine, dissolve it in 100mL ethanol, weigh 7.86g2,2,6, 6-Tetramethyl-4-piperidinol, dissolve it in 100mL N,N-dimethylformamide, mix the two in a 500mL round bottom flask and place it in an ice bath to stir well, add 2g of hydroxide Sodium, react at 15°C for 6h, after the reaction is completed, filter according to the conventional operation method, and wash with distilled water and acetone until the pH value of the filtrate is 7.0, and dry the obtained solid in a vacuum oven at 60°C and 0.2MPa for 12h, Obtain the haloamine antibacterial agent precursor 2-chloro-4-methoxy-6-(2,2,6,6-tetramethylpiperidin-4-oxyl)-1,3,5-triazine, Finally, dissolve the precursor in a hypochlorous acid solution with a concentration of 2.5% by mass, and let it stand at room temperat...
Embodiment 3
[0050] Preparation of halamine antibacterial agent 2,4-difluoro-6-(1-chloro-2,2,6,6-tetramethylpiperidin-4-oxyl)-1,3,5-triazine
[0051] Weigh 6.76g of cyanuric fluoride, dissolve it in 100mL of carbon tetrachloride, weigh 7.86g of 2,2,6,6-tetramethyl-4-piperidinol, dissolve it in 100mL of ethanol, and The two were mixed in a 500mL round-bottomed flask and placed in an ice bath to fully stir evenly. Add 2.8g of potassium hydroxide and react at 25°C for 8 hours. After the reaction was completed, filter according to the conventional operation method, and wash with distilled water and acetone until the filtrate The pH value is 7.0, and the obtained solid is dried in a vacuum oven at 60°C and 0.2 MPa for 12 hours to obtain the precursor of haloamine antibacterial agent 2,4-difluoro-6-(2,2,6,6-tetra Methylpiperidine-4-oxyl)-1,3,5-triazine, and finally dissolve the precursor in a sodium hypochlorite solution with a concentration of 5% by mass, and leave it to react at room temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com