Synthesis method and application of 7-methoxy-3,7-dimethyloctyl aldehyde
A technology of dimethyl octanal and a synthesis method, which is applied to the synthesis method and the application field of the obtained product, can solve the problems such as the influence of the number of varieties and the quality of the product, and achieves the requirements of low production equipment, high yield, and easy availability of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
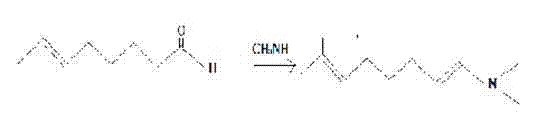
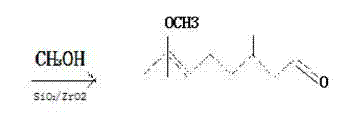
[0020] A kind of 7-methoxy group-3, the synthetic method of 7-dimethyloctanal, reaction in two steps, the first step is intermediate enamine Ⅰ Synthesis, the second step is the synthesis of 7-methoxyl-3,7-dimethyloctanal.
[0021] First step: intermediate enamine Ⅰ The synthesis of dimethylamine deionized water solution with a concentration of 30% was put into the bottle, and the dehydrating agent 4A molecular sieve (the amount was 1% of that of the dimethylamine deionized water solution) was added, the reaction temperature was -5°C, and vigorously stirred Add dropwise 3,7-dimethyl-6-octenal with a concentration of 80% in dimethylamine deionized aqueous solution within 3 hours. After the dropwise addition, continue to react at 20°C for 8 hours. After the reaction is completed, pumping Filter, pour the filtrate into the liquid separation hole, and let it stand for stratification. The lower water layer was separated, the oil layer was fully mixed and washed with saturated sal...
Embodiment 2
[0024] A kind of 7-methoxy group-3,7-dimethyloctanal synthetic method, concrete steps are as follows:
[0025] First step: intermediate enamine Ⅰ The synthesis of dimethylamine deionized water solution with a concentration of 35% was put into the bottle, and an appropriate amount of dehydrating agent 4A molecular sieve (the amount was 1% of the dimethylamine deionized water solution) was added, and the reaction temperature was -10°C. Under stirring, add dropwise 3,7-dimethyl-6-octenal with a concentration of 90% in dimethylamine deionized aqueous solution within 4 hours. After the dropwise addition is completed, continue to react at 25°C for 2 hours. After the reaction is completed, carry out Suction filtration, pour the filtrate into the liquid separation hole, and let it stand for stratification. The lower water layer was separated, the oil layer was fully mixed and washed with saturated salt deionized water, and the lower water layer was separated again. Add an appropria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com