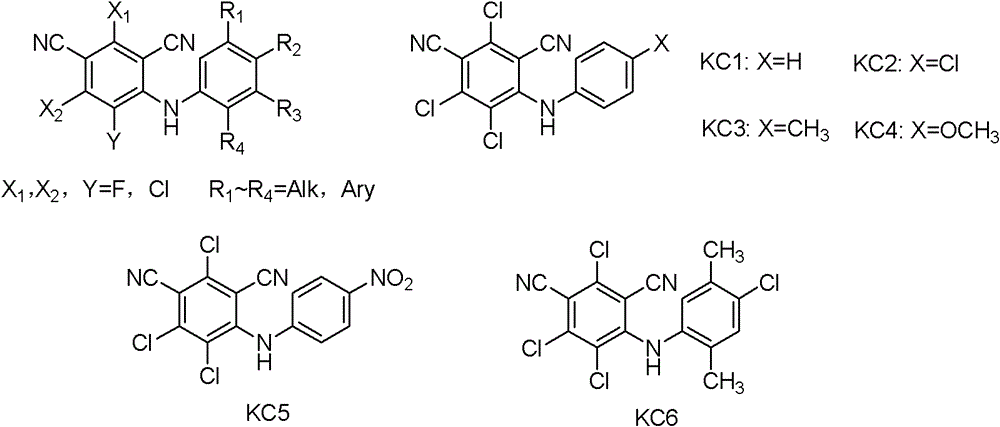
Cyanide-containing diphenylamine compounds and their applications
A technology of cyanodiphenylamine and compounds, which is applied in the field of cyanodiphenylamine-containing compounds, and can solve problems such as unreported structural compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0135] Example 1: Preparation of compound 3
[0136]
[0137] To 1.03g (0.008mol) 2,6-difluoroaniline in 40ml N,N-dimethylformamide solution, add 0.64g (0.016mol) of sodium hydroxide, slowly add 2, 4, 5, 6-Tetrachloro-1,3-phthalonitrile 2.13g (0.008mol), after the addition, continue to stir and react at room temperature for 5h. After the reaction is monitored by TLC, the reaction solution is poured into water, extracted with ethyl acetate, and the organic phase is sequentially After washing with water, washing with saturated brine, drying, filtering, desolventizing, the residue is purified by column chromatography (eluent is ethyl acetate and petroleum ether (boiling range 60-90℃), volume ratio 1:4) to obtain the product 1.65 g, which is compound 3. Light yellow solid, melting point 264-266°C. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 ) δ (ppm): 6.70 (s, 1H, NH), 7.07 (t, 2H, Ph-3, 5-2H, J=8.1 Hz), 7.37 (m, 1H, Ph-4-1H).
example 2
[0138] Example 2: Preparation of compound 4
[0139]
[0140] First put 0.68g (0.002mol) of compound 3 in 20ml concentrated sulfuric acid solution in an ice bath, slowly add the prepared mixed acid (the amount of nitric acid and sulfuric acid are 0.004mol and 0.006mol, respectively) under stirring, and control the temperature at Below 20°C. After the addition is completed, the reaction is continued for 5 min. After the completion of the reaction as monitored by TLC, the reaction solution is poured into ice water and stirred. After cooling to room temperature, extraction with ethyl acetate, the organic phase was washed with saturated brine, dried, filtered, desolventized, and the residue was column chromatographed (eluents are ethyl acetate and petroleum ether (boiling range 60-90°C), The volume ratio is 1:4) 0.40 g of the product was purified, namely compound 4. Light white solid, melting point 204-206°C. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm): 6.70(s, 1...
example 3
[0141] Example 3: Preparation of compound 131
[0142]
[0143] To 10.33g (0.039mol) 4-amino-3,5-dichlorobenzoic acid methyl ester (the preparation method refers to WO2010060379, CN101337940) in 60ml N, N-dimethylformamide solution, add 3.12g sodium hydroxide ( 0.078mol), slowly add 10.37g (0.039mol) of 2,4,5,6-tetrachloro-1,3-phthalonitrile under stirring. After the addition, continue to stir and react at room temperature for 5h. After the reaction is monitored by TLC, The reaction solution was poured into water, extracted with ethyl acetate, the organic phase was washed with water, washed with saturated brine, dried, filtered, desolventized, and the residue was column chromatographed (eluent: ethyl acetate and petroleum ether (boiling range 60- 90°C), the volume ratio is 1:5) 13.65 g of the product, compound 131, was purified. Light yellow solid, melting point 229-231°C. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm): 3.96(s, 3H, CH 3 ), 6.92 (s, 1H, NH), 8.11 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com