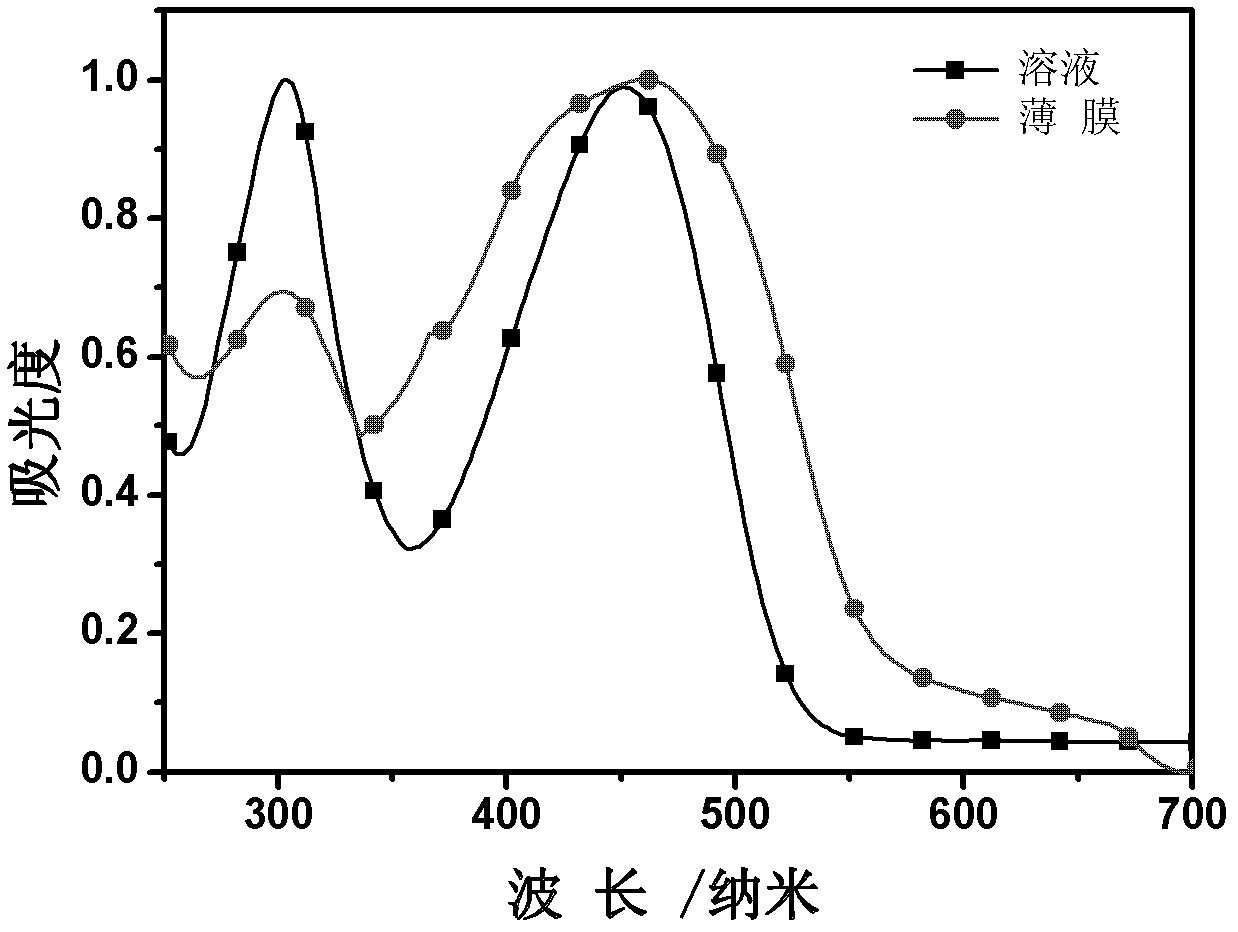
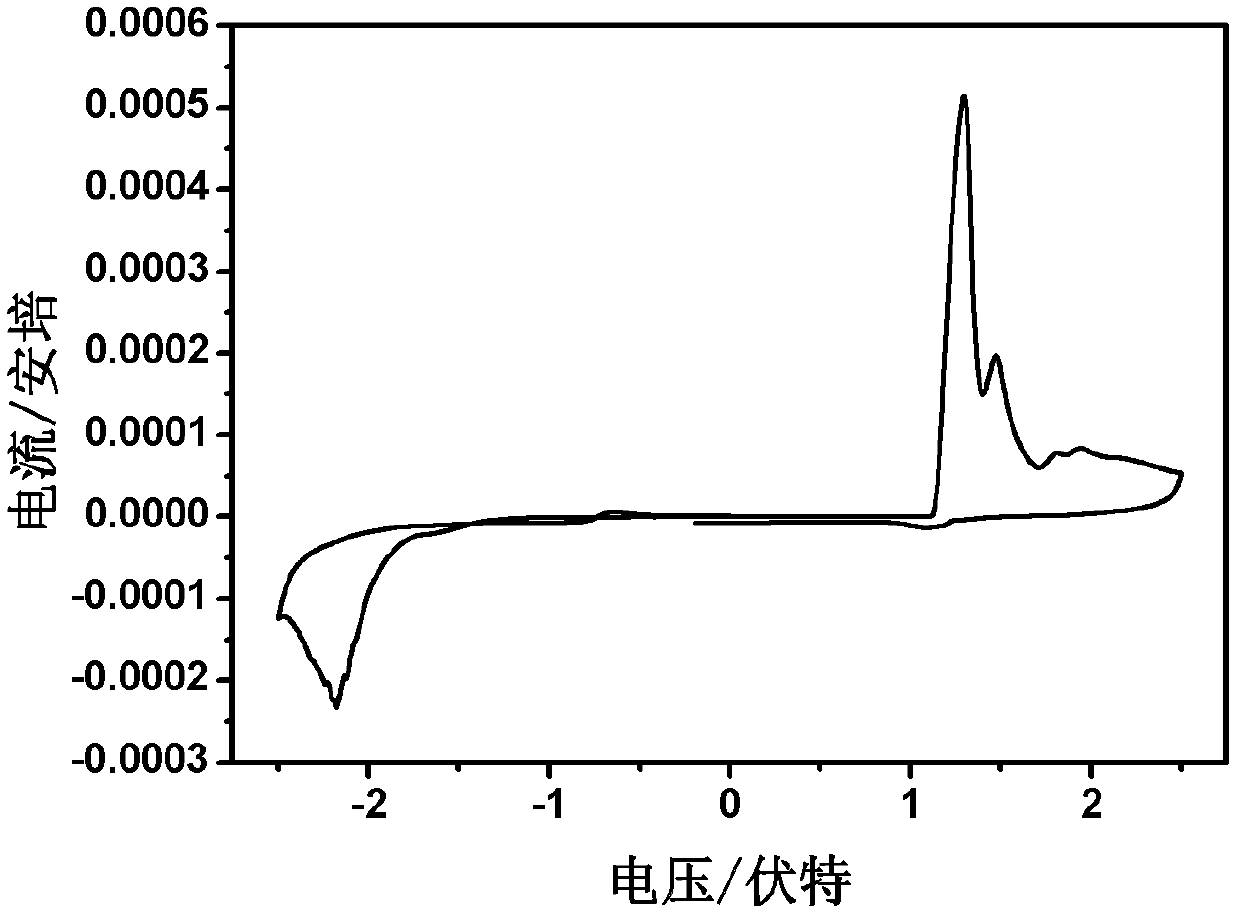
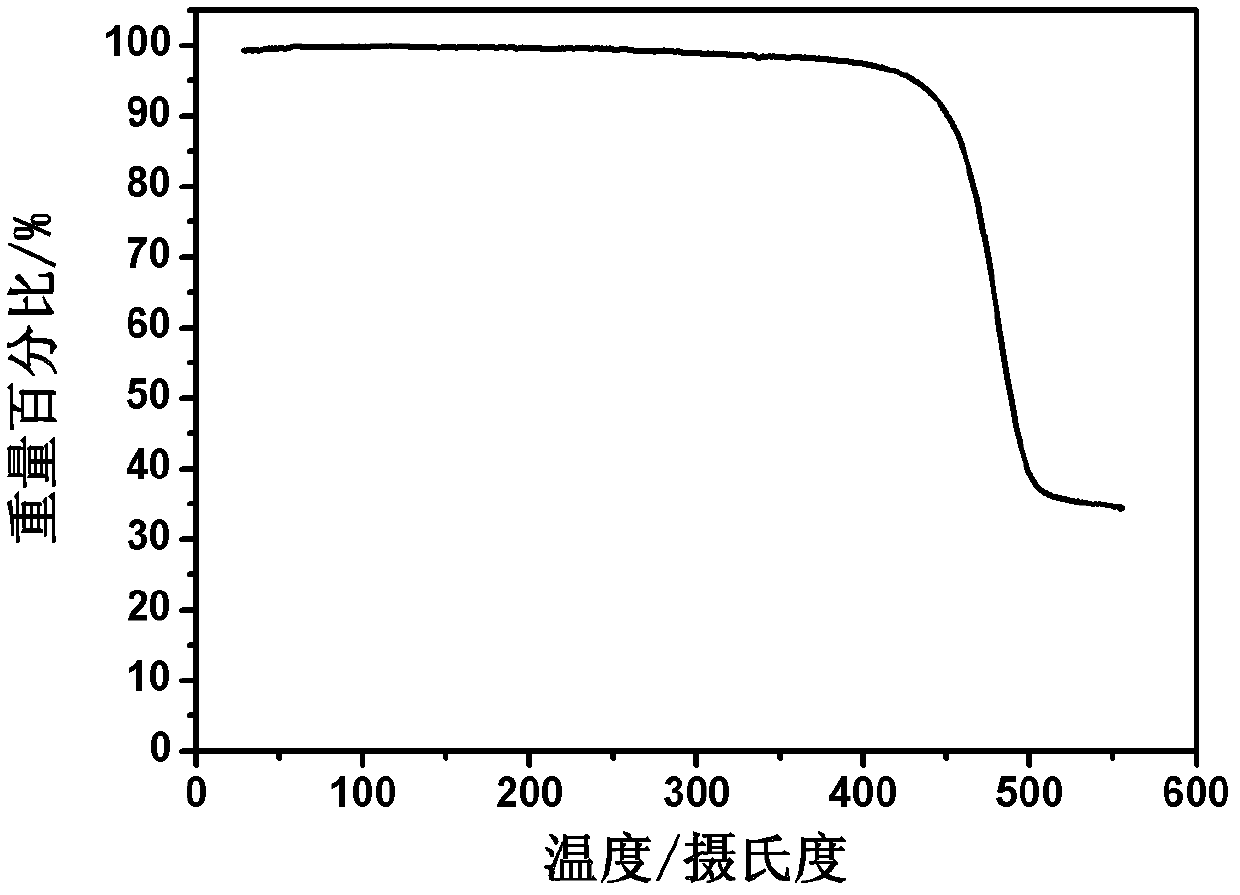
D-A-D conjugated molecule based on triphenylamine-thiophene imide, and preparation method and application thereof
A thiophene imide, D-A-D technology, applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., to achieve good thermal stability, good solubility, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The synthetic route of conjugated molecule 1 is as follows:
[0051]
[0052] Take 65mg (0.13mmol) 1,3-dibromo-5-dodecyl-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione, 230mg (0.4mmol) 4- (3-hexyl-5-(trimethyltin-yl)thiophen-2-yl)-N,N-diphenylaniline in a 25ml three-necked flask, add 5ml of freshly distilled anhydrous toluene, under the protection of nitrogen, stir After 30 minutes, add 20 mg (0.017 mmol) of tetrakis(triphenylphosphine) palladium, heat to 100 ° C, react for 2 days, add 10 ml of KF (5 g) aqueous solution and stir for 2 hours, pour the reaction mixture into water, and extract twice with dichloromethane , combined the organic layers, the obtained organic phase was dried with anhydrous magnesium sulfate, filtered, and the solvent was removed by rotary evaporation to obtain a solid, and the mixed solvent of petroleum ether and dichloromethane in a volume ratio of 4:1 was used as eluent, silica gel ( 200-300 mesh) column chromatography to obtain 120 mg of orange-r...
Embodiment 2
[0055] The synthetic route of conjugated molecule 2 is as follows:
[0056]
[0057] Take 68mg (0.14mmol) of 1,3-dibromo-5-dodecyl-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione, 176mg (0.36mmol) of N, N-diphenyl-4-(5-(trimethyltin)thiophen-2-yl)aniline was placed in a 25ml three-necked flask, 8ml of freshly distilled anhydrous toluene was added, under the protection of nitrogen, stirred for 30min, and four ( Triphenylphosphine)palladium 25mg (0.022mmol), nitrogen gas for 10min, heated to 110°C, reacted for 2 days, added 10ml KF (5g) aqueous solution and stirred for 2 hours, poured the reaction mixture into water, extracted twice with dichloromethane, Combine the organic layers, dry the obtained organic phase with anhydrous magnesium sulfate, filter, remove the solvent by rotary evaporation, use a mixed solvent of petroleum ether and dichloromethane with a volume ratio of 3:1 as eluent, silica gel (200-300 Mesh) column chromatographic separation and purification to obtain 90 mg of ...
Embodiment 3
[0060] The synthetic route of conjugated molecule 3 is as follows:
[0061]
[0062] Take 63mg (0.15mmol) 1,3-dibromo-5-(2-ethylhexyl)-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione, 250mg (0.4mmol )4-(5-(tributyltin-based)thiophen-2-yl)-N,N-diphenylaniline in a 25ml three-necked flask, add 6ml of freshly distilled anhydrous toluene, under the protection of nitrogen, stir for 30min, add Tetrakis(triphenylphosphine)palladium 25mg (0.022mmol), nitrogen gas for 10min, heated to 110°C, reacted for 2 days, added 10ml KF (5g) aqueous solution and stirred for 2 hours, poured the reaction mixture into water, dichloromethane extracted two Once, the organic layers were combined, and the obtained organic phase was dried with anhydrous magnesium sulfate, filtered, and after the solvent was removed by rotary evaporation, the mixed solvent of petroleum ether and dichloromethane with a volume ratio of 3:1 was used as eluent, silica gel (200 -300 mesh) column chromatography separation and purifica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com