Recombinant fusion protein PTD-HSP27 and use thereof
A technology of fusion protein and gene, applied in the field of genetic engineering, to achieve the effect of inhibiting apoptosis, improving the route of medication, and expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
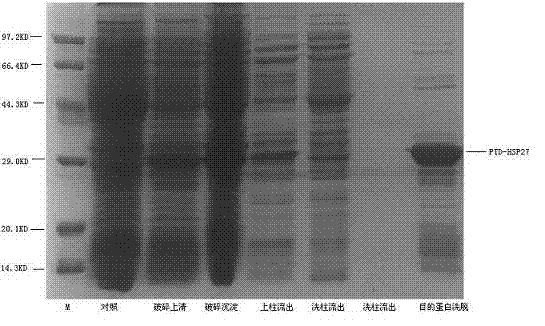
[0026] Example 1 Preparation of recombinant fusion protein PTD-HSP27
[0027] The preparation of the recombinant fusion protein PTD-HSP27 in this example is prepared by conventional gene recombination method.
[0028] (1) Amplification of PTD-HSP27-6HIS gene
[0029] The present invention designs and synthesizes two primers to amplify the PTD-HSP27-6HIS gene (SEQ ID NO: 2) by PCR method. Using primer suspension, PTD short peptide YGRKKRRQRRR was introduced into the N-terminus of the HSP27 gene, and 6 histidine tags were introduced into the C-terminus. On the basis of the existing HSP27 gene, the PTD-HSP27-6His gene was synthesized. The nucleotide sequence of primer 1 is 5'-XXXXXX CATATG TATGGCCGTAAAAAACGTCGTCAGCGTCGTCGTACCGAG CGCCGCGTCCCCTTCTCGCTC-3', the nucleotide sequence of primer 2 is 5'-XXXXXX CTCGAG TTATCAATGATGATGATGATGATGCTTGGCGGCAGTCTCATCGGATT-3', in the above two primers, X represents the protected base, CATATG is the NdeI restriction site, CTCGAG It is ...
Embodiment 2
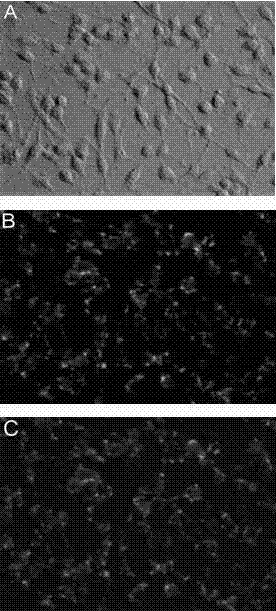
[0040] Example 2 Functional detection of recombinant fusion protein PTD-HSP27 (SEQ ID NO: 1) penetrating cell membrane
[0041] Use fluorescein isothiocyanate (FITC) labeling kit (commercially available) to fluorescently label PTD-HSP27, the steps are as follows:
[0042] 1. FLOUS in the FITC kit is dissolved in DMF (2mg / 0.2ml) to form a fluorescein solution;
[0043] 2. PBS dissolves the fusion protein PTD-HSP27, and the final concentration is ≥4mg / ml;
[0044] 3. Mix the fluorescein and the fusion protein PTD-HSP27 at a ratio of 1:8 (V / V), react in the dark for 2 hours at room temperature, and shake 4 times intermittently;
[0045] 4. After adding 2 ml of PBS, shake the gel column to suspend the deposited gel, fix the gel column upright, and continuously and slowly add 30 ml of PBS to balance the column;
[0046] 5. Pass the above 3 reaction solution through the gel column, elute with 0.8 ml of PBS, discard the eluate, elute with 1 ml of PBS, collect 1 ml of the marker ...
Embodiment 3
[0048] Example 3 Anti-apoptotic effect of recombinant fusion protein PTD-HSP27 (SEQ ID NO: 1) on human lens epithelial cells
[0049] (1) UV irradiation LEC time screening
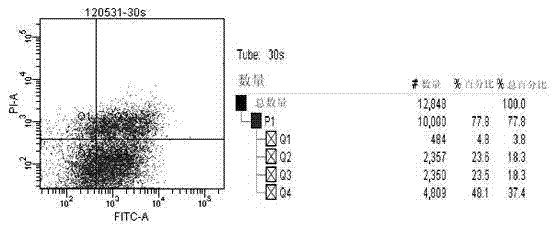
[0050] Human LEC cells in the logarithmic growth phase were taken, and the cell culture medium was aspirated before irradiation, and washed once with PBS. Placed at 10 cm under the ultraviolet light source, the irradiation time was 0s, 5s, 10s, 20s, 30s, 1min. Cell apoptosis rate was detected by flow cytometry ( image 3 ), suggesting that the apoptosis rate of LEC cells was close to half after 30s of ultraviolet irradiation, and the ultraviolet irradiation time of 30s was chosen as the unified irradiation time for subsequent experiments.
[0051] (2) Screening of optimal incubation concentration of recombinant fusion protein PTD-HSP27
[0052] Prepare 20× PTD-HSP27 recombinant fusion protein stock solution with a concentration of 10 μM (ie 0.27 mg / ml). The protein stocks were diluted respectively to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com