Synthesis method of pyridylacetonitrile
A technology of pyridineacetonitrile and synthesis method, applied in directions such as organic chemistry, can solve problems such as complicated post-processing, expensive raw materials, etc., and achieve the effects of stable operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
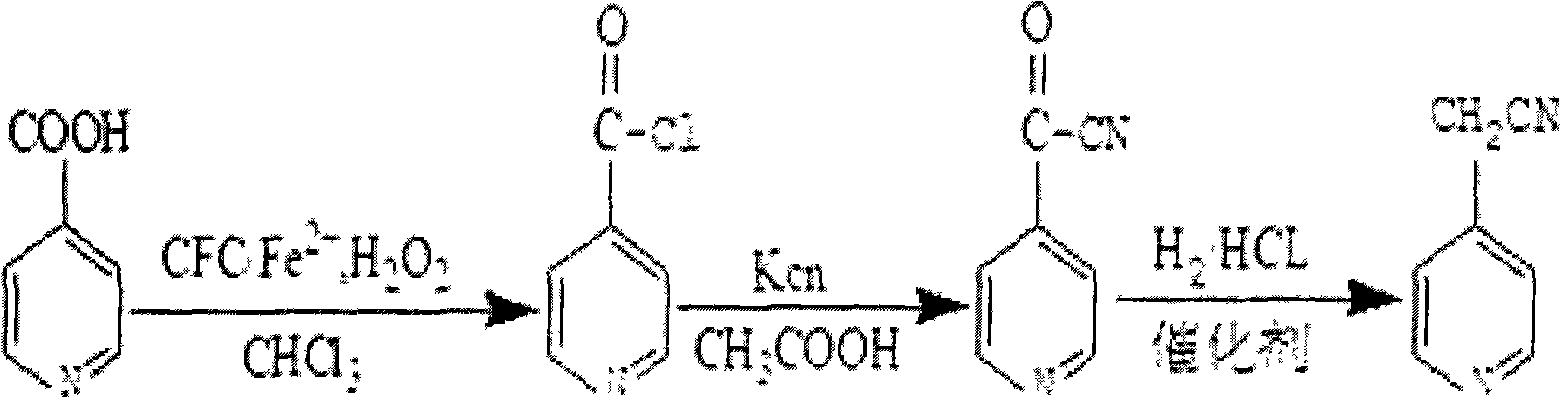
[0020] 2-pyridinecarboxylic acid 3.62g (0.58mol) difluorodichloromethane (CFC-12) 3.62g (0.58mol) methyl
[0021] Iodine 3.62g (0.58mol) Hydrogen oxide solution 162.3g (1.45mol)
[0022] Ferrous chloride 162.3g (1.45mol)
[0023] Its synthetic method is:
[0024] Add 2-pyridinecarboxylic acid, difluorodichloromethane (CFC-12), and methyl iodide into a 520ml three-necked flask, dissolve in 200ml chloroform, add hydrogen peroxide solution and chlorination solution dropwise under ice-water cooling For ferrous ferrous, keep the temperature of the reaction solution not exceeding 7°C, gradually raise it to 28°C after the dropwise addition, stir for 2 hours, heat up and reflux for about 3 hours, and precipitate the solid by suction filtration, and the solid obtained after the filtrate recovers the solvent is combined into a product, and the solid product is Freely obtain picolinoyl chloride; then dissolve picolinoyl chloride in 483ml, 65% acid ester solution, add potassium cyanide,...
example 2
[0026] 3-pyridinecarboxylic acid 2.82g (0.36mol) a fluorotrichloromethane (CFC-11) 2.93g (0.45mol) methyl
[0027] Bromine 3.62g (0.58mol) Hydrogen oxide solution 169.3g (1.49mol)
[0028] Ferrous chloride 162.3g (1.45mol)
[0029] Its synthetic method is:
[0030] Add 3-pyridinecarboxylic acid, fluorotrichloromethane (CFC-11), and methyl bromide into a 520ml three-necked flask, dissolve in 200ml of chloroform, add hydrogen peroxide solution and chlorinated For ferrous ferrous, keep the temperature of the reaction solution not exceeding 7°C, gradually raise it to 25°C after the dropwise addition, stir for 2 hours, heat up and reflux for about 2.5 hours, and precipitate the solid by suction filtration, and the solid obtained after the filtrate recovers the solvent is combined into a product, and the solid Free the product to obtain picolinoyl chloride; then dissolve picolinoyl chloride in 483ml, 65% acid ester solution, add potassium cyanide, heat to 81°C, and reflux for 1h. ...
example 3
[0032] 4-pyridinecarboxylic acid 3.62g (0.58mol) methyl chloride 3.62g (0.58mol)
[0033] Methyl Bromide 4.08g (0.69mol) Hydrogen Oxide Solution 165.7g (1.51mol)
[0034] Ferrous chloride 162.3g (1.45mol)
[0035] Its synthetic method is:
[0036] Add 4-pyridinecarboxylic acid, methyl chloride, and methyl bromide into a 496ml three-necked flask, dissolve in 200ml of chloroform, add hydrogen peroxide solution and ferrous chloride dropwise under ice water cooling, and keep the temperature of the reaction solution Not more than 7°C, gradually raised to 25°C after the dropwise addition, stirred for 2 hours, heated and refluxed for about 2.5 hours, the solid was precipitated by suction filtration, and the solid obtained after the filtrate recovered the solvent was combined into a product, and the solid product was freed to obtain picolinoyl chloride; Then dissolve picolinoyl chloride in 483ml, 65% acid vinegar solution, add potassium cyanide, heat to 81°C, and reflux for 1h. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com