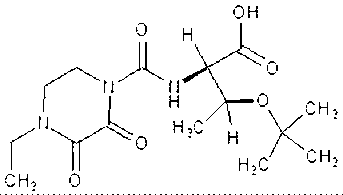
Preparation method of cefbuperazone side chain
A technology of cefbuperazone and side chains, applied in the field of preparation of cefbuperazone side chains, can solve the problems of large amount of solvent used, complex process operation, high reaction cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of side chains:
[0040] In a dry 250ml three-necked flask, add 7.4g of D-threonine and 80ml of anhydrous DCM, stir, and pass through the nitrogen replacement system. Add 10 ml of trimethylchlorosilane dropwise at room temperature, cool down to 0°C in an ice bath, slowly add 12 ml of triethylamine dropwise, control the dropping temperature at 0°C, and naturally warm up to room temperature after dropping, and react for 2 hours. After the reaction was completed, the temperature was cooled to 0°C in an ice bath, and 20ml of anhydrous DCM solution of 12g of EDPC was added dropwise at a temperature of 5°C.
[0041] Post-reaction treatment: suction filtration, washing the filter cake with DCM. The filtrate was washed with 20ml of water for 30min and separated. The organic phase was adjusted to pH 8.0 with saturated sodium carbonate solution. Separate the liquids, wash the organic phase twice with 40ml saturated sodium bicarbonate solution, 5min each time, and co...
Embodiment 2
[0043] Synthesis of side chains
[0044] In a dry 250ml three-necked flask, add 15g of D-threonine and 162ml of anhydrous DCM, stir, and pass through the nitrogen replacement system. Add 20 ml of trimethylchlorosilane dropwise at room temperature, cool down to 5°C in an ice bath, slowly add 24 ml of triethylamine dropwise, and control the dropwise temperature at 10°C, then naturally warm up to room temperature after dropping, and react for 2 hours. After the reaction was completed, the temperature was cooled to 0°C in an ice bath, and 40ml of anhydrous DCM solution of 24.3g of EDPC was added dropwise at a temperature of 10°C.
[0045] Post-reaction treatment: suction filtration, washing the filter cake with DCM. The filtrate was washed with 40ml of water for 30min and separated. The organic phase was adjusted to pH 7.0 with saturated sodium carbonate solution. Separate the liquids, wash the organic phase with 80ml of saturated sodium bicarbonate solution twice for 5min ea...
Embodiment 3
[0047] Synthesis of side chains
[0048] In a dry 250ml three-necked flask, add 20g of D-threonine and 216ml of anhydrous DCM, stir, and pass through the nitrogen replacement system. Add 27 ml of trimethylchlorosilane dropwise at room temperature, cool down to 2°C in an ice bath, slowly add 32.4 ml of triethylamine dropwise, and control the dropping temperature at 5°C, then naturally warm up to room temperature after dropping, and react for 2 hours. After the reaction was completed, the temperature was cooled to 0°C in an ice bath, and 54ml of anhydrous DCM solution of 32.4g of EDPC was added dropwise at a temperature of 6°C.
[0049]Post-reaction treatment: suction filtration, washing the filter cake with DCM. The filtrate was washed with 54ml of water for 30min and separated. The organic phase was adjusted to pH 10.0 with saturated sodium carbonate solution. Separate the liquids, wash the organic phase with 108ml of saturated sodium bicarbonate solution twice for 5min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com