Application of chrysalis oil in improving small molecule drug bioavailability
A technology of silkworm chrysalis oil and small molecules, which is applied in the field of biomedicine, can solve the problems of low absorption, side effects, and slow effect, and achieve the effects of increased drug absorption concentration, wide application prospects, and strong improvement ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
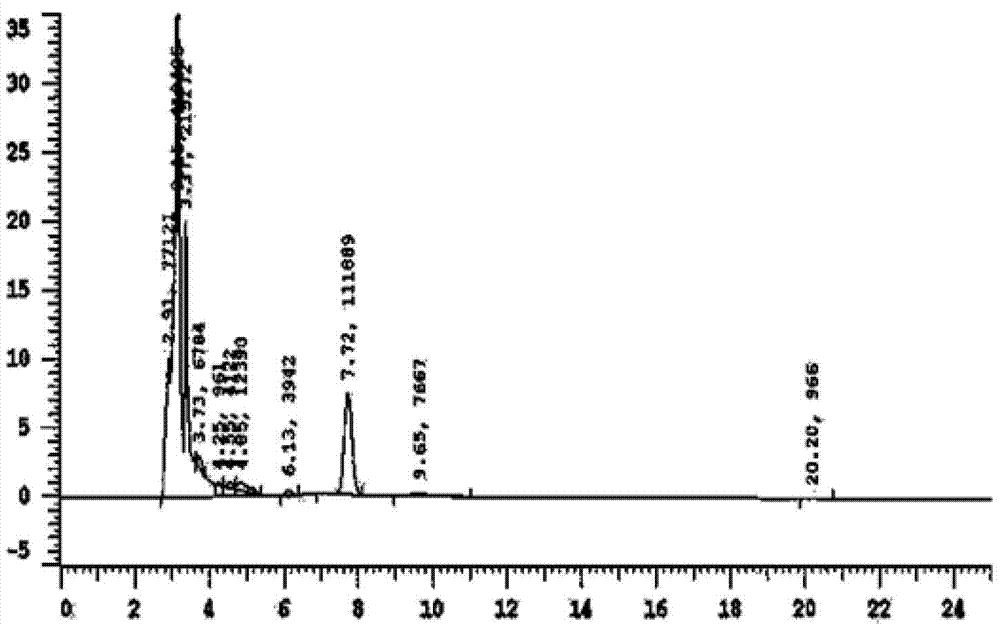
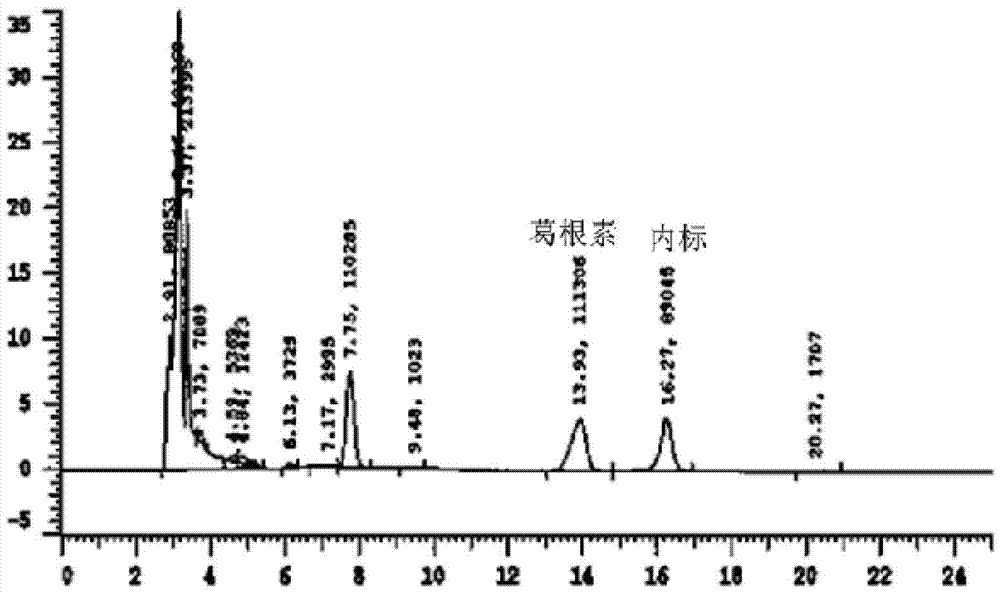
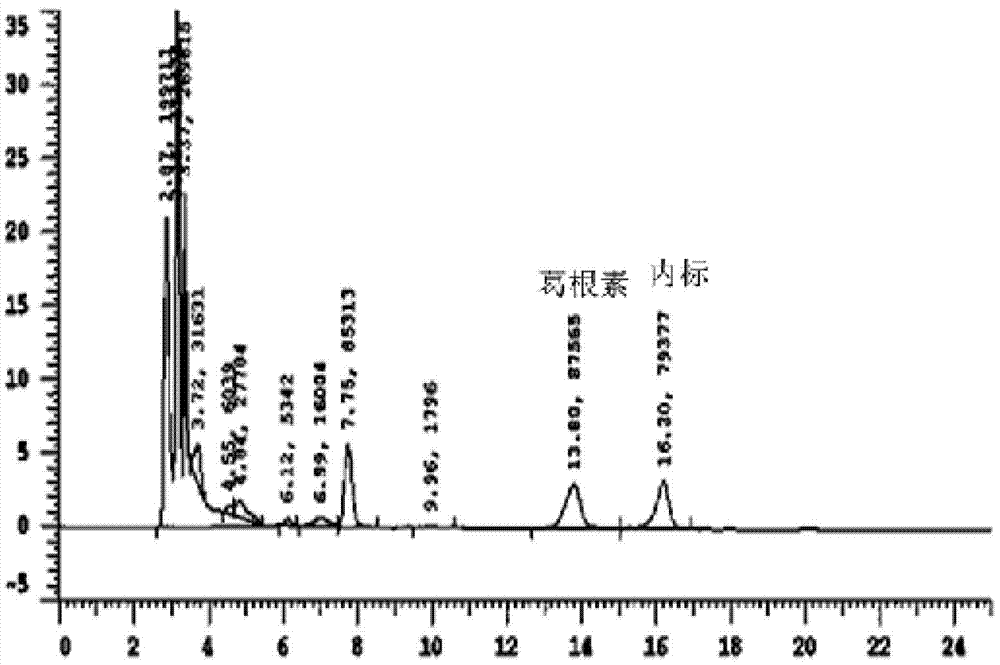
[0023] Example 1: Exploration of liquid phase conditions and selection of internal standard
[0024] Chromatographic column Venusil XBP C18 (4.6mm×250mm, 5m); the mobile phase is methanol: water (30:70) (v / v); the flow rate is 0.7ml / min, the column temperature is 30°C; the ultraviolet detection wavelength is 250nm; Amount of 20l internal standard is 4-hydroxybenzaldehyde.
Embodiment 2
[0025] Embodiment 2: Serum processing and determination
[0026] After the blood was collected, the mice were kept still at room temperature for 30 minutes, centrifuged at 3500 rpm at 4°C for 10 minutes, and 190 μl of the supernatant was taken into a 1.5ml EP tube, 10 μl of 4-hydroxybenzaldehyde internal standard solution (100 μg / ml) was added and shaken to mix, and 400 μl of acetonitrile was added. Vortex for 5 minutes, centrifuge at 12,000 rpm for 10 minutes, take the supernatant and pass it through a 0.22 μm organic membrane, and load 20 μl of the sample.
Embodiment 3
[0027] Embodiment 3: the preparation of standard stock solution and standard curve
[0028] Accurately weigh 10 mg of puerarin standard substance, put it in a 10ml volumetric flask, add methanol to dissolve and dilute to the mark, shake well, and then obtain 1mg / ml internal standard stock solution; then dilute to concentrations of 5, 10, 25, 50 , 100, and 225 μg / ml of puerarin methanol series standard solutions, respectively get 10 μl and add in 190 μl serum, add 10 μl 4-hydroxybenzaldehyde internal standard solution (100 μg / ml) and shake and mix well, and the processing method is the same as that in "Example 2 Serum Processing and Determination", HPLC detection, with the blood drug concentration C of puerarin as the abscissa, and the peak area ratio A of puerarin as the ordinate, the standard curve equation is A=0.0014C+0.076, at 83ng / ml~3750ng / ml Good linearity in the range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com